Question: 1. Solid State Diffusion Gorilla R Glass by Corning is found in many touchscreens on devices such as smartphones because it offers superior scratch resistance
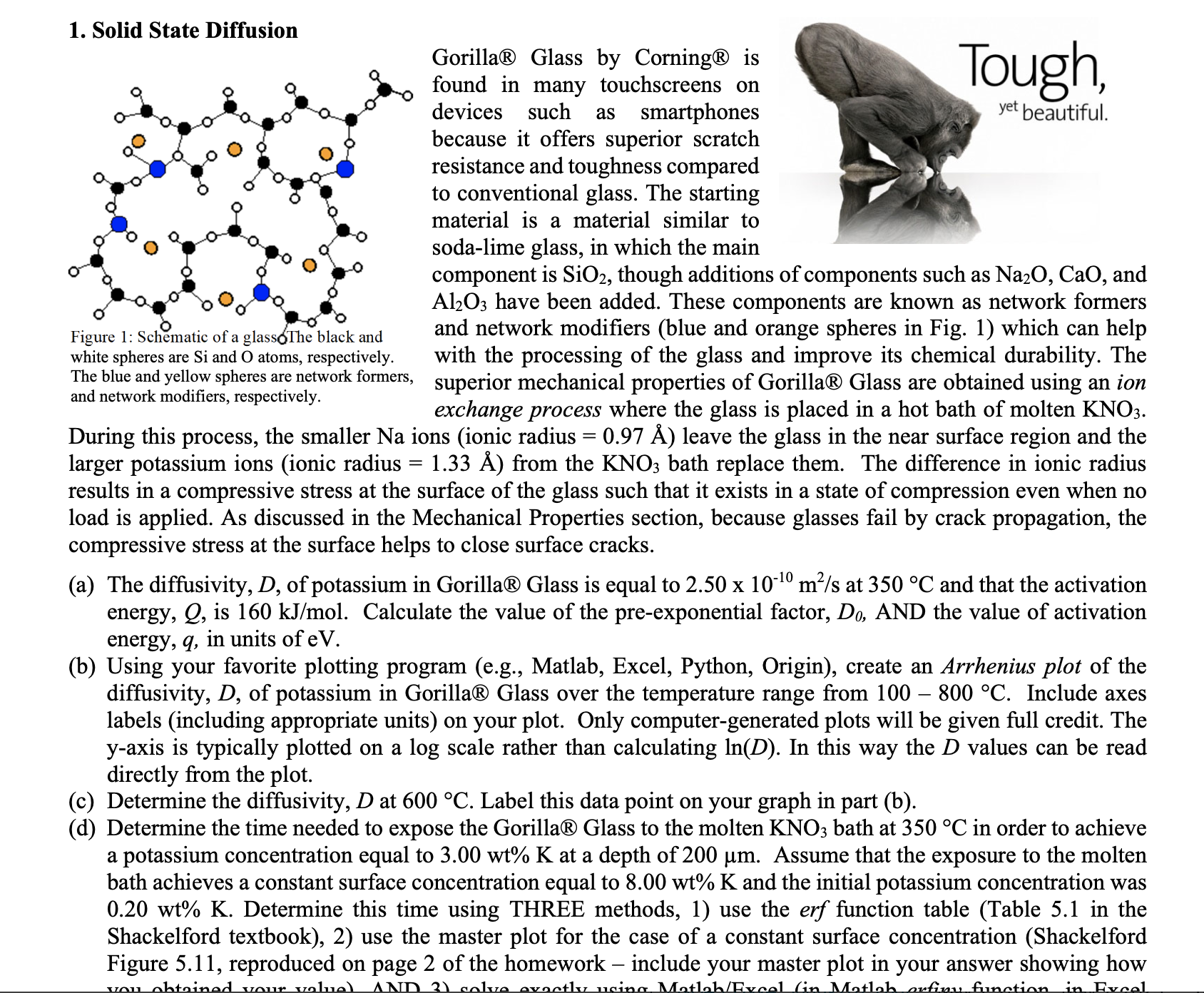
1. Solid State Diffusion Gorilla R Glass by Corning is found in many touchscreens on devices such as smartphones because it offers superior scratch resistance and toughness compared to conventional glass. The starting material is a material similar to soda-lime glass, in which the main component is SiO2, though additions of components such as Na2O,CaO, and Al2O3 have been added. These components are known as network formers Figure 1: Schmatic of a glassolhe black and white spheres are Si and O atoms, respectively. The blue and yellow spheres are network formers, and network modifiers, respectively. and network modifiers (blue and orange spheres in Fig. 1) which can help with the processing of the glass and improve its chemical durability. The superior mechanical properties of Gorilla R Glass are obtained using an ion exchange process where the glass is placed in a hot bath of molten KNO3. During this process, the smaller Na ions (ionic radius =0.97A ) leave the glass in the near surface region and the larger potassium ions (ionic radius =1.33A ) from the KNO3 bath replace them. The difference in ionic radius results in a compressive stress at the surface of the glass such that it exists in a state of compression even when no load is applied. As discussed in the Mechanical Properties section, because glasses fail by crack propagation, the compressive stress at the surface helps to close surface cracks. (a) The diffusivity, D, of potassium in Gorilla R Glass is equal to 2.501010m2/s at 350C and that the activation energy, Q, is 160kJ/mol. Calculate the value of the pre-exponential factor, D0, AND the value of activation energy, q, in units of eV. (b) Using your favorite plotting program (e.g., Matlab, Excel, Python, Origin), create an Arrhenius plot of the diffusivity, D, of potassium in Gorilla Glass over the temperature range from 100800C. Include axes labels (including appropriate units) on your plot. Only computer-generated plots will be given full credit. The y-axis is typically plotted on a log scale rather than calculating ln(D). In this way the D values can be read directly from the plot. (c) Determine the diffusivity, D at 600C. Label this data point on your graph in part (b). (d) Determine the time needed to expose the Gorilla Glass to the molten KNO3 bath at 350C in order to achieve a potassium concentration equal to 3.00wt%K at a depth of 200m. Assume that the exposure to the molten bath achieves a constant surface concentration equal to 8.00wt%K and the initial potassium concentration was 0.20wt%K. Determine this time using THREE methods, 1) use the erf function table (Table 5.1 in the Shackelford textbook), 2) use the master plot for the case of a constant surface concentration (Shackelford Figure 5.11, reproduced on page 2 of the homework - include your master plot in your answer showing how
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


