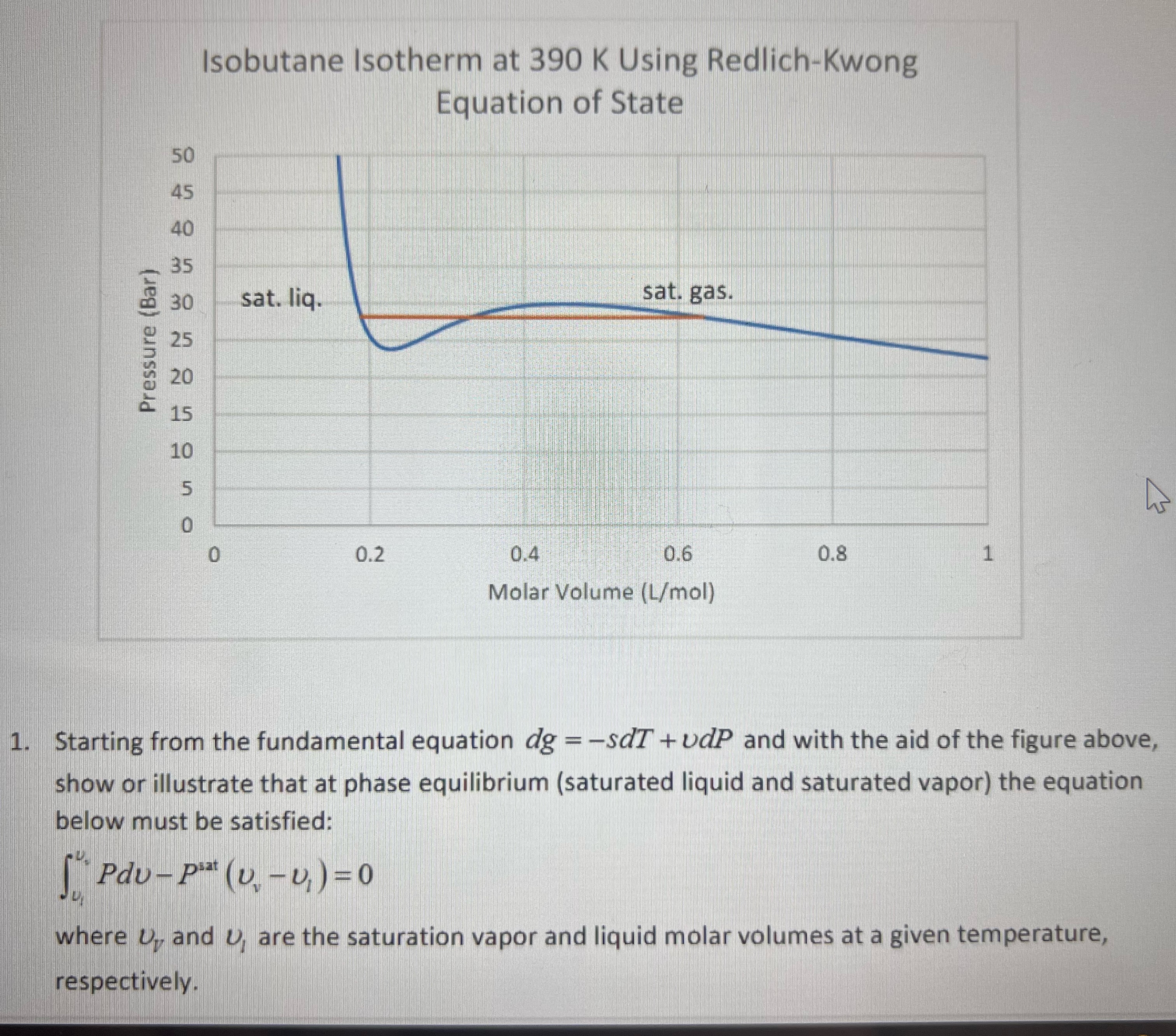
Question: 1 . Starting from the fundamental equation dg = - sdT + dP and with the aid of the figure above,show or illustrate that at
Starting from the fundamental equation dg sdT dP and with the aid of the figure above,show or illustrate that at phase equilibrium saturated liquid and saturated vapor the equation below must be satisfied:pdv psat UvU
where U and U are the saturation vapor and liquid molar volumes at a given temperature, respectively.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


