Question: 1. To standardize the KMnO4 solution, a redox titration is performed. The MnO4ions react with a known quantity of Fe? ions. Here are the two
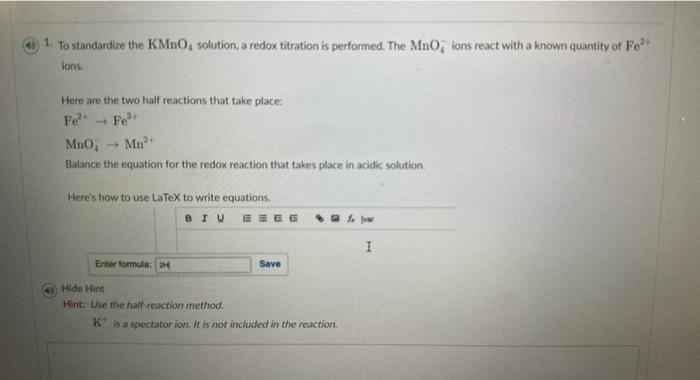
1. To standardize the KMnO4 solution, a redox titration is performed. The MnO4ions react with a known quantity of Fe? ions. Here are the two half reactions that take place: Fe2+Fe3+MnO4Mn2+ Balance the equation for the redox reaction that takes place in acidic solution. Here's how to use LaTeX to write equations. (4) Hide Hint Hint: Use the haif-reaction method. K+is a spectator ion. It is not included in the reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


