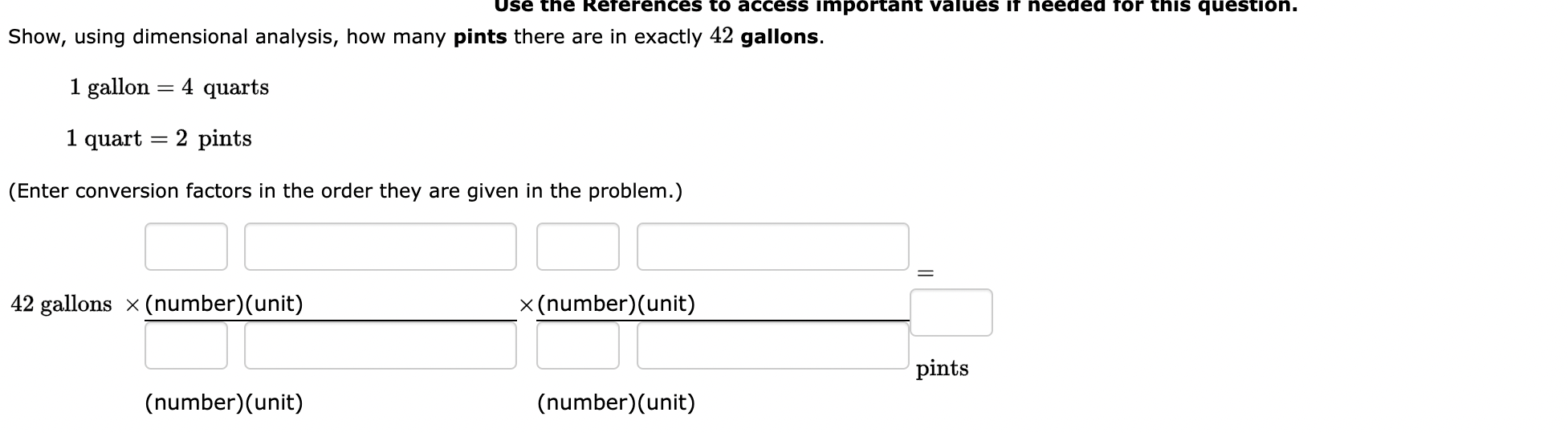
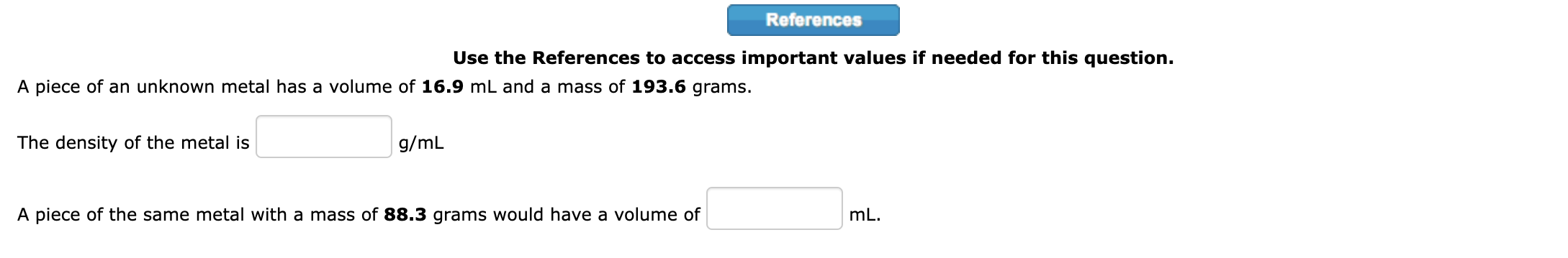
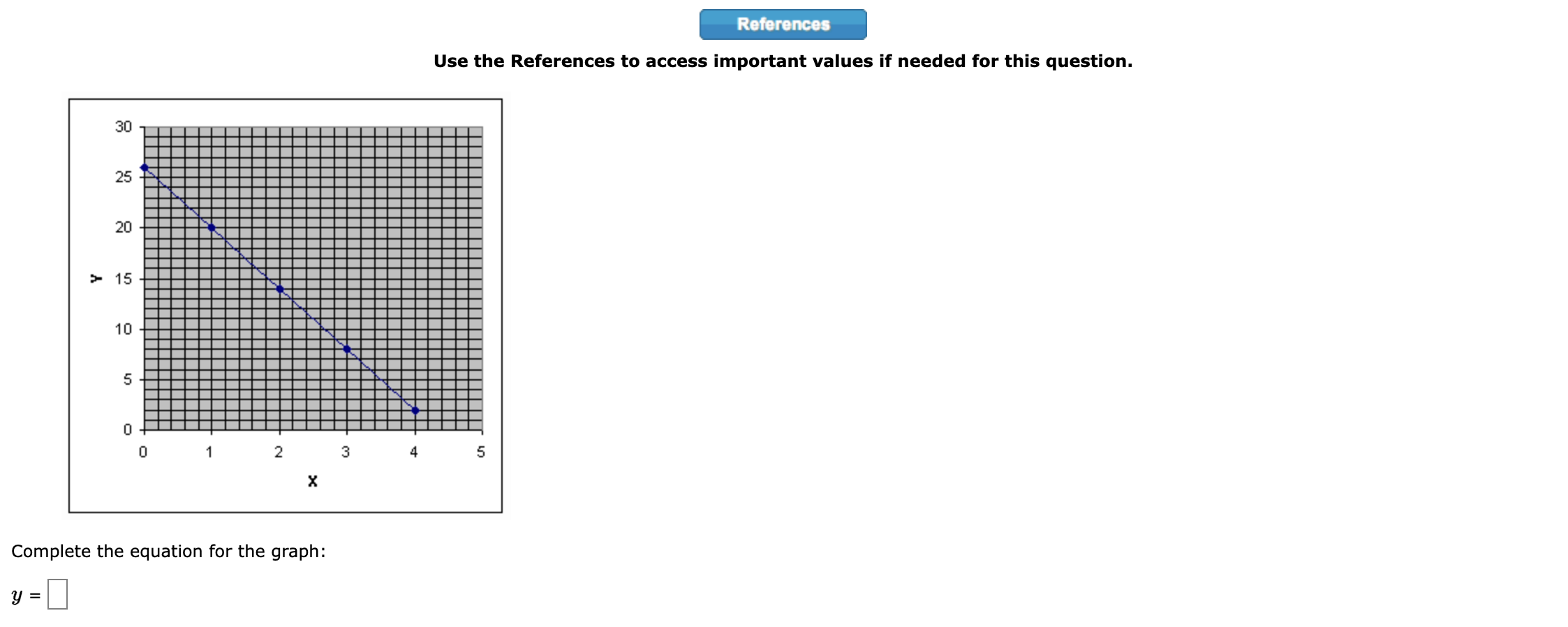
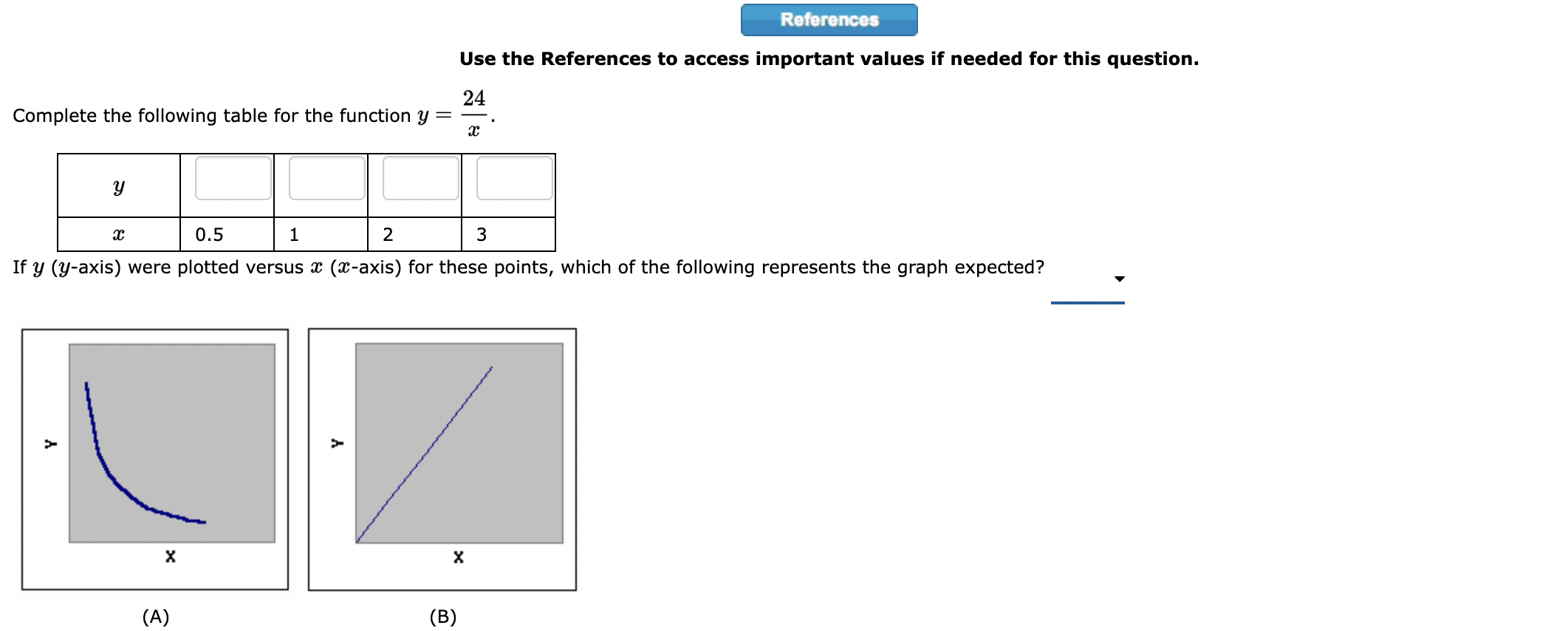
Question: 1. Use the References to access important values if needed for this question. Show, using dimensional analysis, how many pints there are in exactly 42
1.
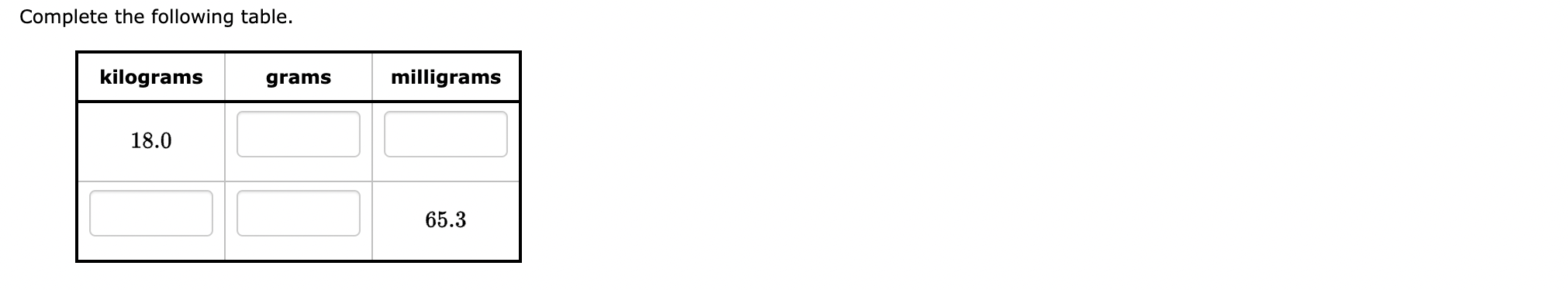







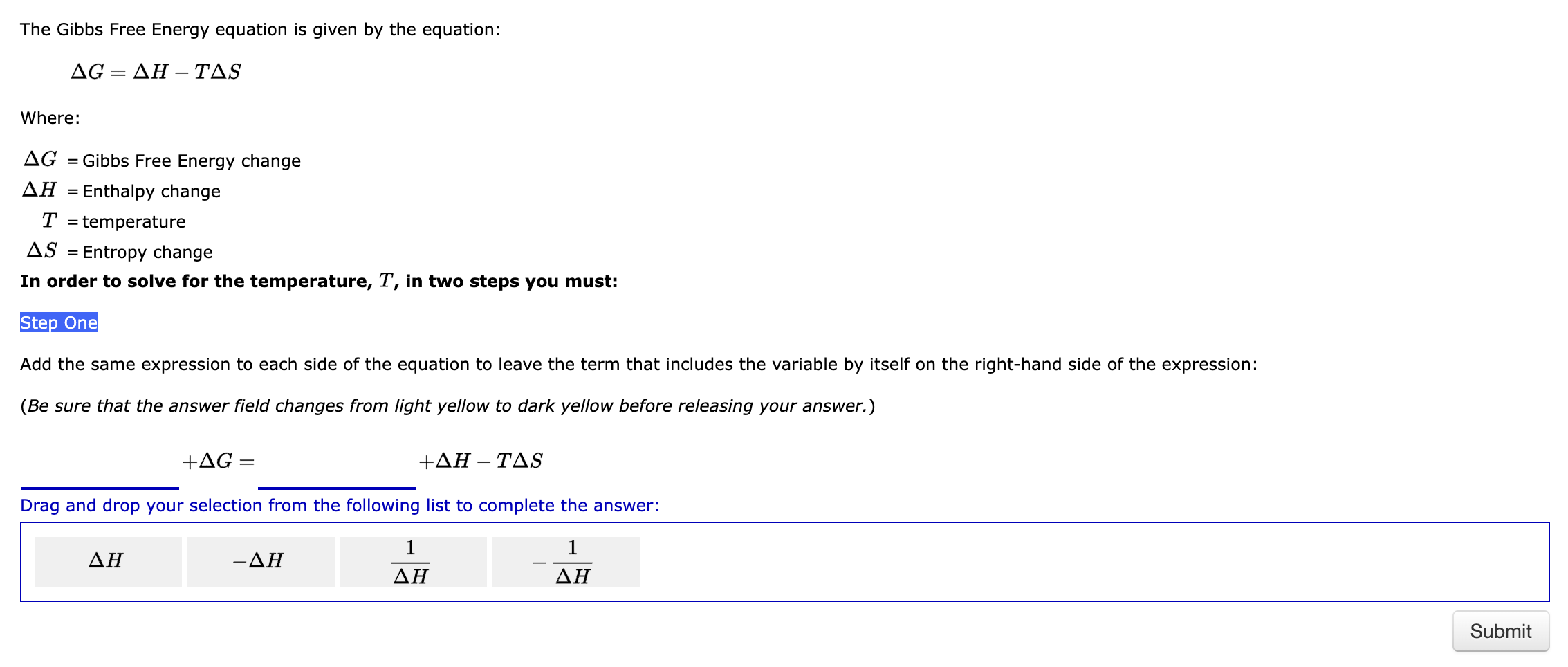
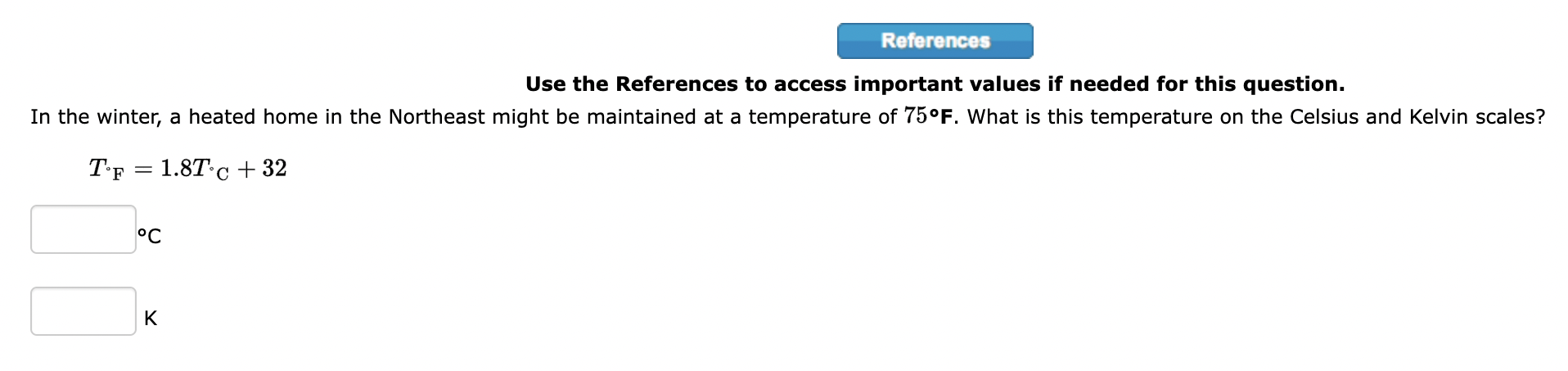

Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


