Question: 1. Using data in Appendix C, calculate H,S, and G at 298K for each of the following reactions. Indicate whether the reaction is spontaneous at
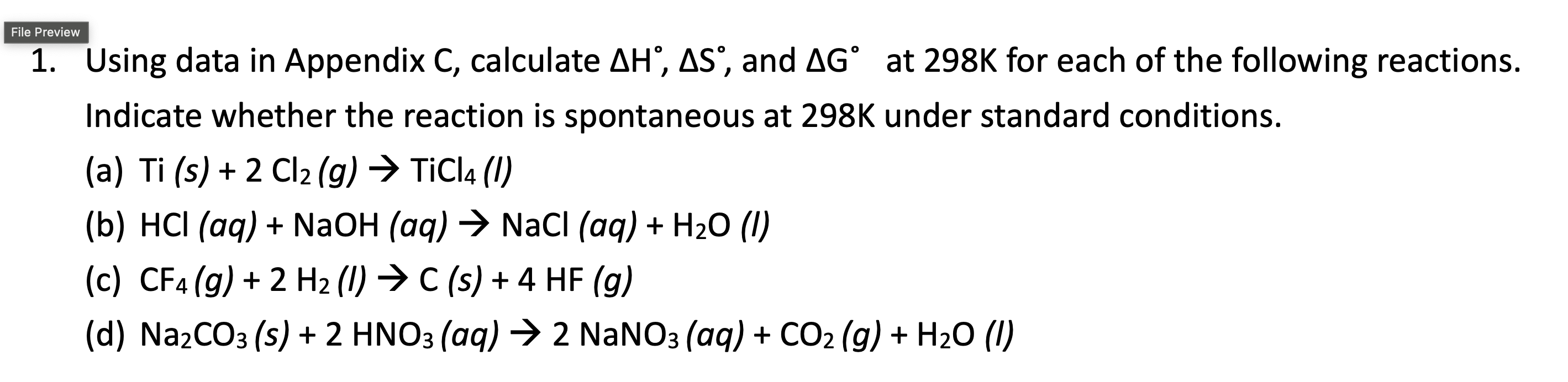
1. Using data in Appendix C, calculate H,S, and G at 298K for each of the following reactions. Indicate whether the reaction is spontaneous at 298K under standard conditions. (a) Ti(s)+2Cl2(g)TiCl4(I) (b) HCl(aq)+NaOH(aq)NaCl(aq)+H2O (l) (c) CF4(g)+2H2(l)C(s)+4HF(g) (d) Na2CO3(s)+2HNO3( aq )2NaNO3(aq)+CO2(g)+H2O (l) 1. Using data in Appendix C, calculate H,S, and G at 298K for each of the following reactions. Indicate whether the reaction is spontaneous at 298K under standard conditions. (a) Ti(s)+2Cl2(g)TiCl4(I) (b) HCl(aq)+NaOH(aq)NaCl(aq)+H2O (l) (c) CF4(g)+2H2(l)C(s)+4HF(g) (d) Na2CO3(s)+2HNO3( aq )2NaNO3(aq)+CO2(g)+H2O (l)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


