Question: 1. Using the Arrhenius Equation. Given that the rate constant for the H + O2 OH + O reaction is 4.7 x 1010 cmmol-1 s-1
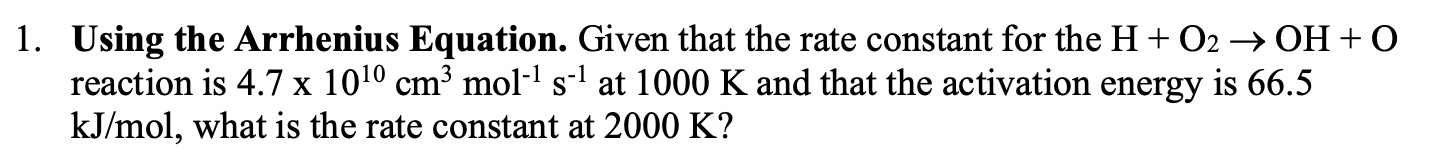
1. Using the Arrhenius Equation. Given that the rate constant for the H + O2 OH + O reaction is 4.7 x 1010 cmmol-1 s-1 at 1000 K and that the activation energy is 66.5 kJ/mol, what is the rate constant at 2000 K? X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


