Question: 1. Were there any experimental errors or design flaws that may have caused a low percentage yield? 2. Are there any experimental errors/reasons that might
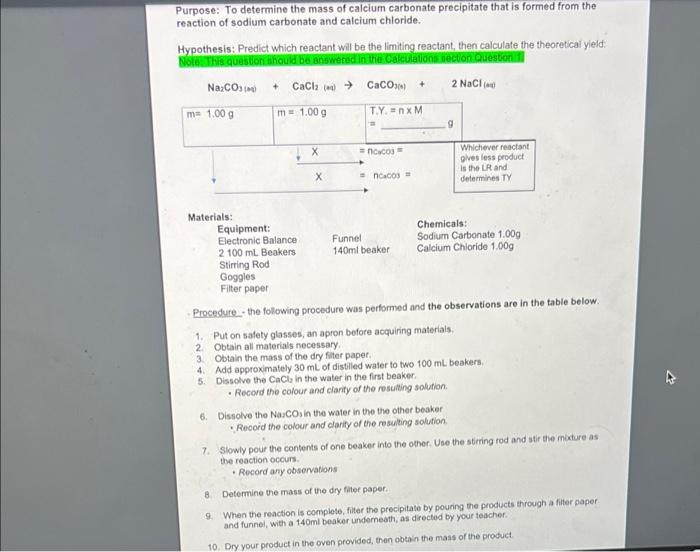
Purpose: To determine the mass of calcium carbonate precipitate that is formed from the reaction of sodium carbonate and calcium chloride. Hypothesis: Predict which reactant will be the limiting reactant, then calculate the theoretical yield. Note: This question should be answered in the Calculation section Question Na:COM CaCl in Caco + 2 NaCl me 1.000 m= 1.009 TYxM g =ncos Whichever reactant glves less product is the LR and determines TY naco Materials: Equipment: Chemicals: Electronic Balance Funnel Sodium Carbonate 1.000 2 100 mL Beakers 140ml beaker Calcium Chloride 1.009 Stirring Rod Goggles Filter paper Procedure_- the following procedure was performed and the observations are in the table below. 1. Put on safety glasses, an apron before acquiring materials, 2 Obtain all materials necessary 3. Obtain the mass of the dry filter paper 4 Add approximately 30 mL of distiled water to two 100 ml beakers 5. Dissolve the Cach in the water in the first beaker Record the colour and clarity of the resulting solution 6. Dissolve the Nascos in the water in the the other beaker Record the colour and clarity of the resulting solution 7. Slowly pour the contents of one beaker into the other. Use the string rod and stir the mixture as the reaction cours Record any observations 8 Determine the mass of the dry filter paper 9 When the reaction is complete, filter the precipitate by pouring the products through after paper and funnel, with a 140ml beaker underneath, as directed by your teacher. 10. Dry your product in the oven provided, then obtain the mass of the product
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


