Question: 1. What is the average (rms) speed of the molecules of a helium gas at a temperature of 21C? m/s 2. A steel-belted radial automobile

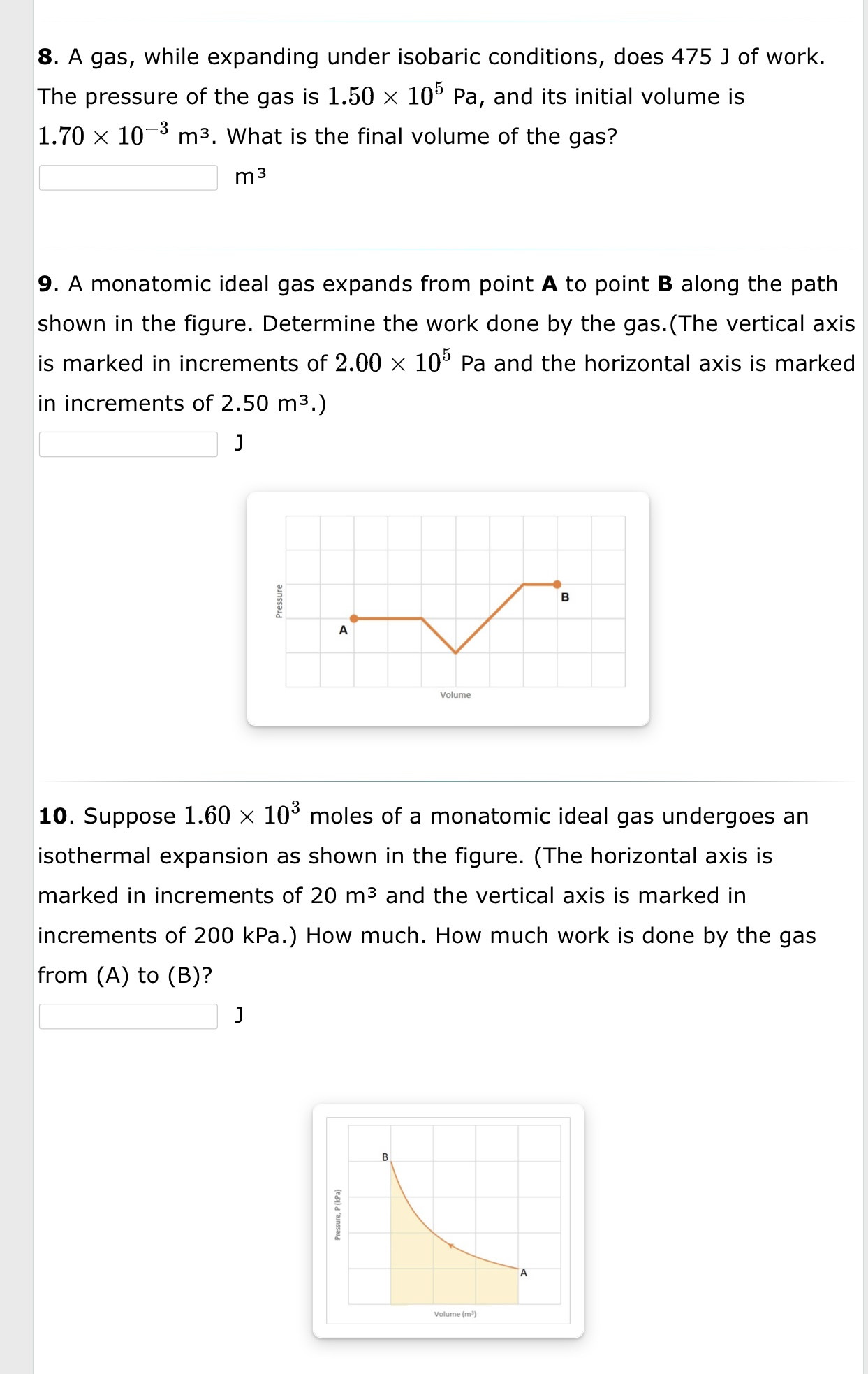
1. What is the average (rms) speed of the molecules of a helium gas at a temperature of 21C? m/s 2. A steel-belted radial automobile tire is inflated to a gauge pressure of 2.00 x 105 Pa when the temperature is 64 F. Later in the day, the temperature rises to 104 F. Assuming the volume of the tire remains constant, what is the gauge pressure at the elevated temperature? [Hint: Remember that the ideal gas law uses absolute pressure.] Pa 3. A Goodyear blimp typically contains 5400 m3 of helium (He) at an absolute pressure of 1.20 x 105 Pa. The temperature of the helium is 293 K. What is the mass (in kg) of the helium in the blimp?(The molar mass of Helium is 4.0026 g) kg 4. A rigid tank contains 65.0 g of chlorine gas (Clz) at a temperature of 76 C and an absolute pressure of 5.90 X 105 Pa. Later, the temperature of the tank has dropped to 35 C and, due to a leak, the pressure has dropped to 3.60 x 105 Pa. How many grams of chlorine gas have leaked out of the tank? (The mass per mole of Clz is 70.9 g/mol.) 9 5. Four moles of an ideal monatomic gas are at a temperature of 330 K. Then, 2470 J of heat is added to the gas, and 900 J of work is done on it. What is the final temperature of the gas? K 6. Heat is added isothermally to 2.6 mol of a monatomic ideal gas. The temperature of the gas is 440 K. How much heat must be added to make the volume of the gas quadruple? J 7. A monatomic ideal gas in a rigid container is heated from 34 C to 96 C by adding 8.90 X 104 J of heat. How many moles of gas are there in the container? moles 8. A gas, while expanding under isobaric conditions, does 475 J of work. The pressure of the gas is 1.50 x 105 Pa, and its initial volume is 1.70 X 103 m3. What is the final volume of the gas? m3 9. A monatomic ideal gas expands from point A to point B along the path shown in the gure. Determine the work done by the gas.(The vertical axis is marked in increments of 2.00 X 105 Pa and the horizontal axis is marked in increments of 2.50 m3.) J g B E A Volume 10. Suppose 1.60 x 103 moles of a monatomic ideal gas undergoes an isothermal expansion as shown in the figure. (The horizontal axis is marked in increments of 20 m3 and the vertical axis is marked in increments of 200 kPa.) How much. How much work is done by the gas from (A) to (B)? Prawn. F l in
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


