Question: 1. What type (redox, acid-base, precipitation) are the reactions below? For the redox reactions, identify the element that is oxidized and reduced, as well as
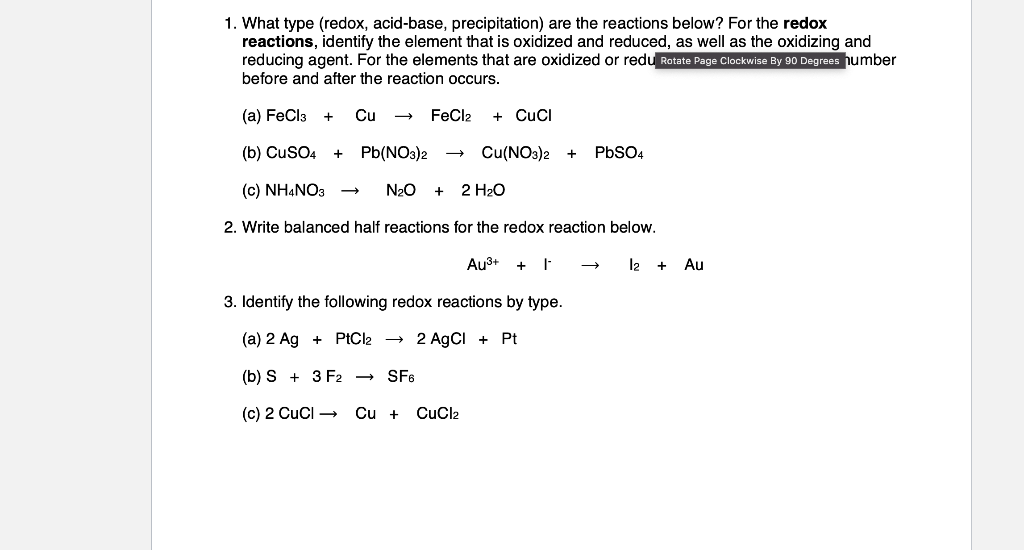
1. What type (redox, acid-base, precipitation) are the reactions below? For the redox reactions, identify the element that is oxidized and reduced, as well as the oxidizing and reducing agent. For the elements that are oxidized or redul Rotate Page Clockwise By 90 Degrees humber before and after the reaction occurs. (a) FeCl3+CuFeCl2+CuCl (b) CuSO4+Pb(NO3)2Cu(NO3)2+PbSO4 (c) NH4NO3N2O+2H2O 2. Write balanced half reactions for the redox reaction below. Au3++FI2+Au 3. Identify the following redox reactions by type. (a) 2Ag+PtCl22AgCl+Pt (b) S+3F2SF6 (c) 2CuClCu+CuCl2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


