Question: 1. Why is KS ionic while HS is molecular? What factors must you consider in answering this question? 2. Complete the Lewis structures for the
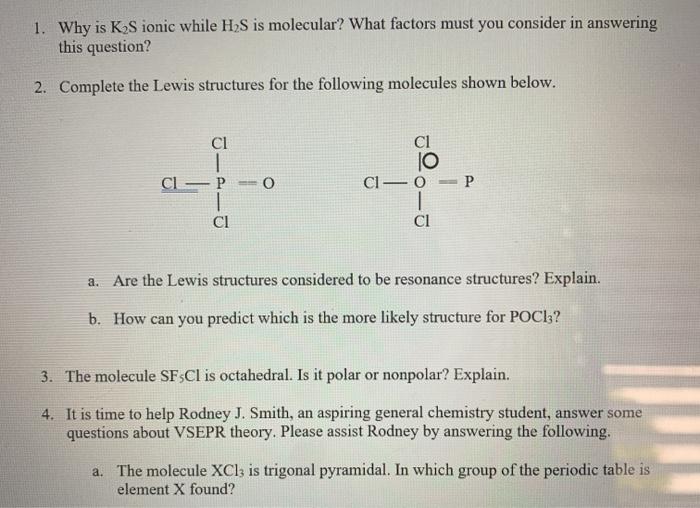

1. Why is KS ionic while HS is molecular? What factors must you consider in answering this question? 2. Complete the Lewis structures for the following molecules shown below. ci ci CL - P -O P CI-O 1 CI CI a. Are the Lewis structures considered to be resonance structures? Explain. b. How can you predict which is the more likely structure for POCI3? 3. The molecule SF3Cl is octahedral. Is it polar or nonpolar? Explain. 4. It is time to help Rodney J. Smith, an aspiring general chemistry student, answer some questions about VSEPR theory. Please assist Rodney by answering the following. a. The molecule XClz is trigonal pyramidal. In which group of the periodic table is element X found? 4. It is time to help Rodney J. Smith, an aspiring general chemistry student, answer some questions about VSEPR theory. Please assist Rodney by answering the following. a. The molecule XCl3 is trigonal pyramidal. In which group of the periodic table is element X found? b. If the molecule were trigonal planar, in which group would X be located? c. If the molecule were T-shaped, in which group would X reside
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


