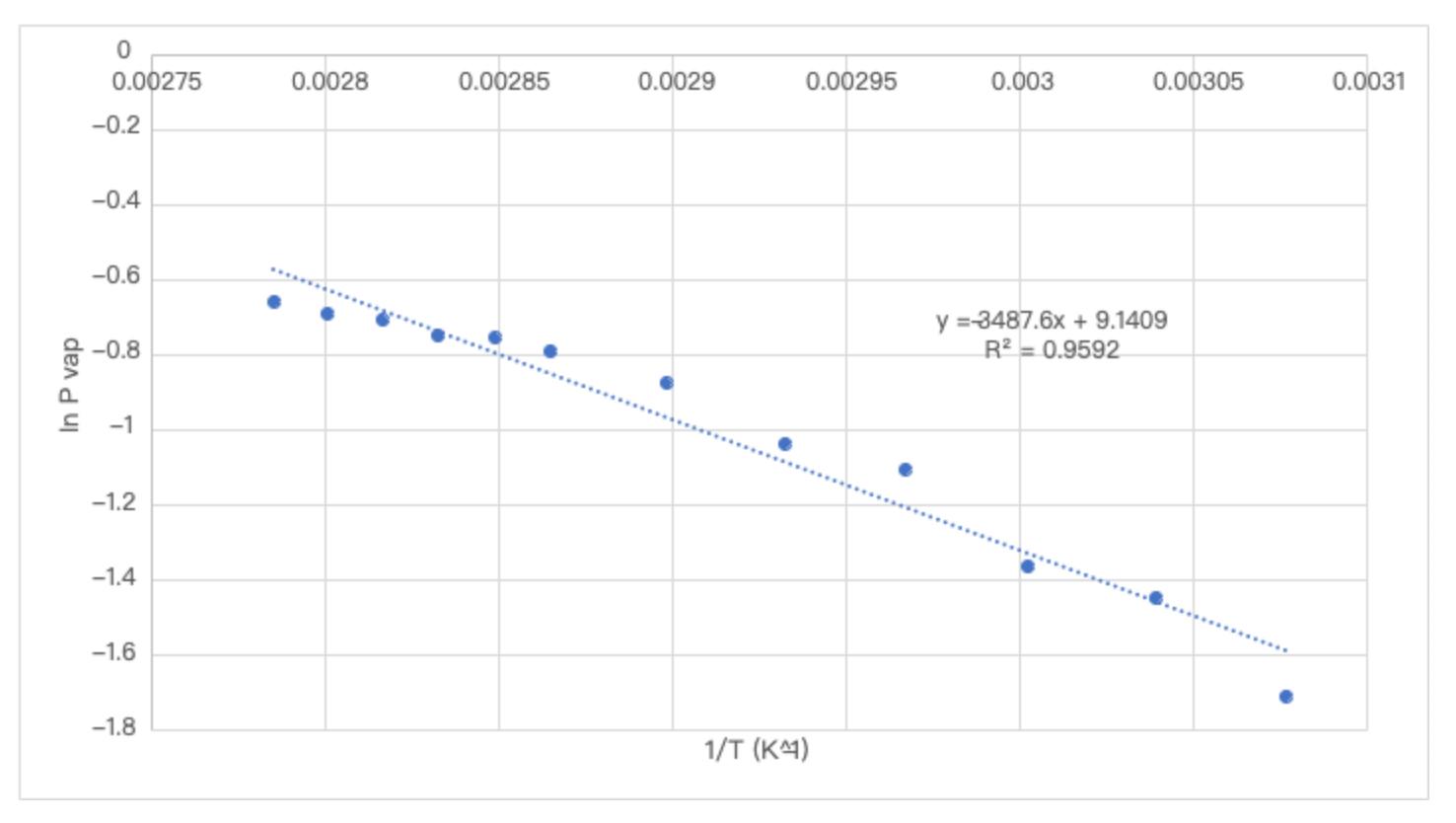
Question: 1 . Would you expect the Hvap for pentane ( CH 3 CH 2 CH 2 CH 2 CH 3 ) to be higher or
Would you expect the Hvap for pentane CHCHCHCHCH to be higher or lower than that of water? Why?
Would you expect the addition of a small amount of a salt the water to increase or decrease the vapor pressure readings at each temperature? If you are unsure, think about whether the solution would have higher or lower IMFs than the pure water, and what that would do to the Hvap for the solution compared to pure water. Other things to think about are Henrys law and the effect of a solute on boiling point. How might dirty glassware affect your results? talk about this as a source of error
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


