Question: 1. Write down the electron configurations for the following ions: P+2+, Pb2+, Rut, Cu2+, Zr, and 02. (6 points) 2. Calculate the force of attraction
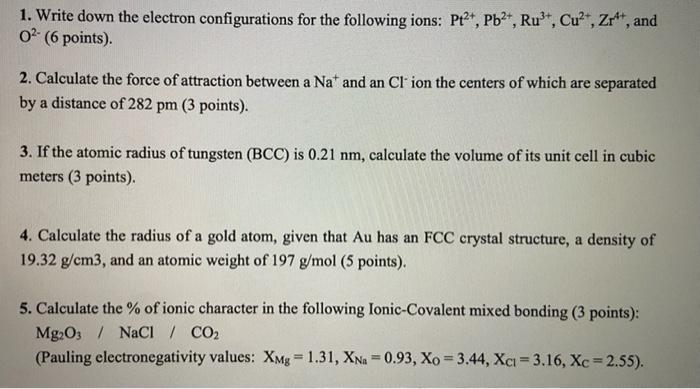
1. Write down the electron configurations for the following ions: P+2+, Pb2+, Rut, Cu2+, Zr, and 02. (6 points) 2. Calculate the force of attraction between a Nat and an Cl' ion the centers of which are separated by a distance of 282 pm (3 points). 3. If the atomic radius of tungsten (BCC) is 0.21 nm, calculate the volume of its unit cell in cubic meters (3 points) 4. Calculate the radius of a gold atom, given that Au has an FCC crystal structure, a density of 19.32 g/cm3, and an atomic weight of 197 g/mol (5 points). 5. Calculate the % of ionic character in the following Ionic-Covalent mixed bonding (3 points): Mg2O3 / NaCl / CO2 (Pauling electronegativity values: XMg = 1.31, XNa=0.93, Xo = 3.44, Xcu = 3.16, Xc = 2.55)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


