Question: 1. Write the equilibrium constant expression for the cobalt equilibrium . 2. Calculate the Keq of the cobalt equilibrium before and after each stressor was
1. Write the equilibrium constant expression for the cobalt equilibrium
. 2. Calculate the Keq of the cobalt equilibrium before and after each stressor was added. Record your answer in the data table. Include a sample calculation in this section
. 3. What direction did the equilibrium shift when you added HCl? What evidence do you have to support this? Explain the shift using Le Chateliers principle. '
4. What direction did the equilibrium shift when you added AgNO3? What evidence do you have to support this? What evidence do you have to support this? Explain the shift using Le Chateliers principle.
5. How did the equilibrium shift when the system was diluted? What evidence do you have to support this? Explain the shift using Le Chateliers principle.
6. Was this reversible reaction exothermic or endothermic? How do you know? Explain using Le Chateliers principle.
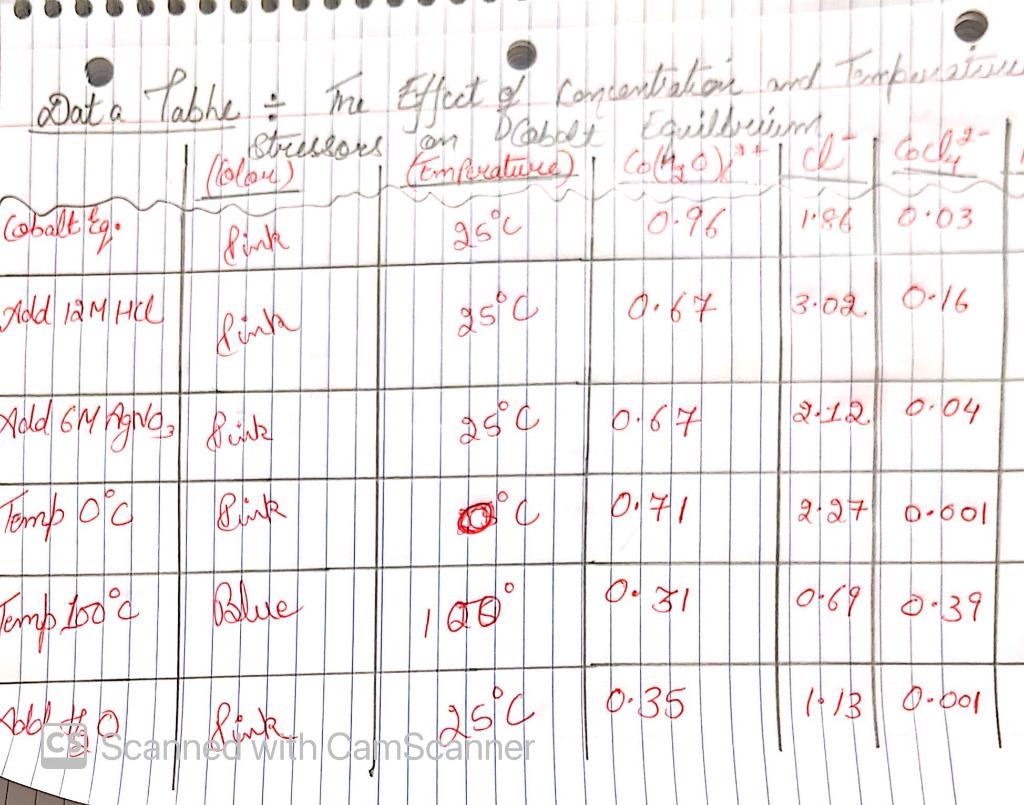
7. Did the Keq value for the equilibrium system exhibit any reasonable change as a result of the stresses you applied? If so, which stresses caused a change in the Keq value?
Dat a Table & me Effect of concentration and Exporate con Strelots Polietileno y lo pode coco Cemperatuee? COM Cobalt Ego Add 12 M He () 0.97 poh 6.03 1.86 link 1959 25d 0.67 link 3.02 0.16 Add 6M Agro Pink 34 6.67 2.12 0.04 Tomp ord Blink 0.71 g9 00601 0.31 10-691 0.39 Temp bood Blue 20 boleh Scarinderia cambd2541 0.35 1 B1 0.001
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


