Question: 1) Write the missing reactants or products. (16p) d) - PCC OH OH Dry dichloromethane b) OH . 2 OH c) PCC OH ? h)
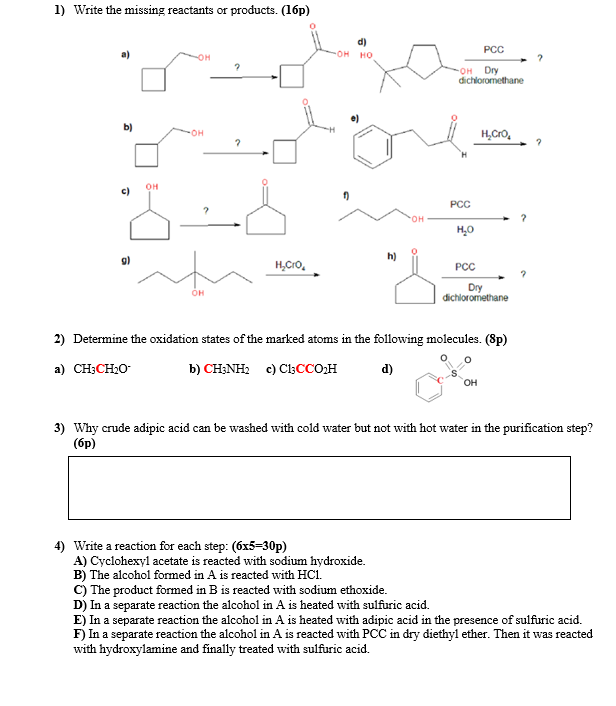
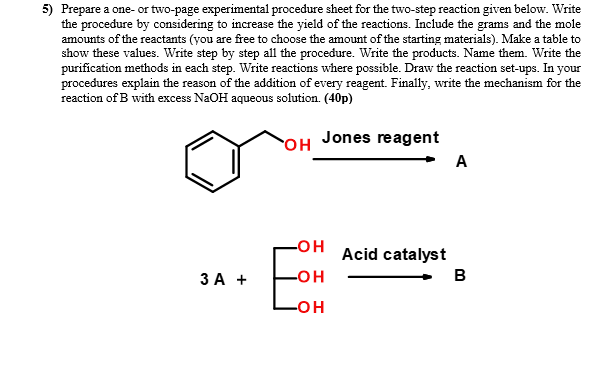
1) Write the missing reactants or products. (16p) d) - PCC OH OH Dry dichloromethane b) OH . 2 OH c) PCC OH ? h) H.CO. PCC OH Dry dichloromethane 2) Determine the oxidation states of the marked atoms in the following molecules. (Sp) a) CH3CH20 b) CH3NH2 c) C13CCO H d) OH 3) Why crude adipic acid can be washed with cold water but not with hot water in the purification step? (6p) 4) Write a reaction for each step: (6x5=30p) A) Cyclohexyl acetate is reacted with sodium hydroxide. B) The alcohol formed in A is reacted with HCl. C) The product formed in B is reacted with sodium ethoxide. D) In a separate reaction the alcohol in A is heated with sulfuric acid. E) In a separate reaction the alcohol in A is heated with adipic acid in the presence of sulfuric acid. F) In a separate reaction the alcohol in A is reacted with PCC in dry diethyl ether. Then it was reacted with hydroxylamine and finally treated with sulfuric acid. 5) Prepare a one or two-page experimental procedure sheet for the two-step reaction given below. Write the procedure by considering to increase the yield of the reactions. Include the grams and the mole amounts of the reactants (you are free to choose the amount of the starting materials). Make a table to show these values. Write step by step all the procedure. Write the products. Name them. Write the purification methods in each step. Write reactions where possible. Draw the reaction set-ups. In your procedures explain the reason of the addition of every reagent. Finally, write the mechanism for the reaction of B with excess NaOH aqueous solution. (40p) OH Jones reagent A -OH Acid catalyst B 3 A + -OH B -OH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


