Question: 10. (3 points): We will make the following solution in class. Calculate the volume or amount of each component needed to make 10mL of the
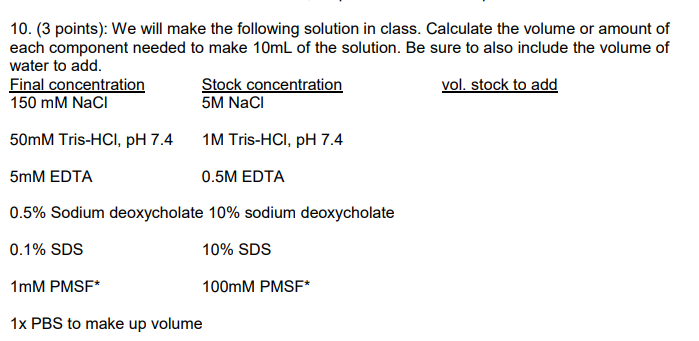
10. (3 points): We will make the following solution in class. Calculate the volume or amount of each component needed to make 10mL of the solution. Be sure to also include the volume of water to add. Final concentration Stock concentration vol. stock to add 150 mM NaCl 5M Naci 50mM Tris-HCl, pH 7.4 1M Tris-HCl, pH 7.4 5mM EDTA 0.5M EDTA 0.5% Sodium deoxycholate 10% sodium deoxycholate 0.1% SDS 10% SDS 1mM PMSF* 100mM PMSF* 1x PBS to make up volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


