Question: 10. For each compound, fil in the table with the electronic geometry, molecular geomelry, and hybridization for the atom indicated with an arrow - assume
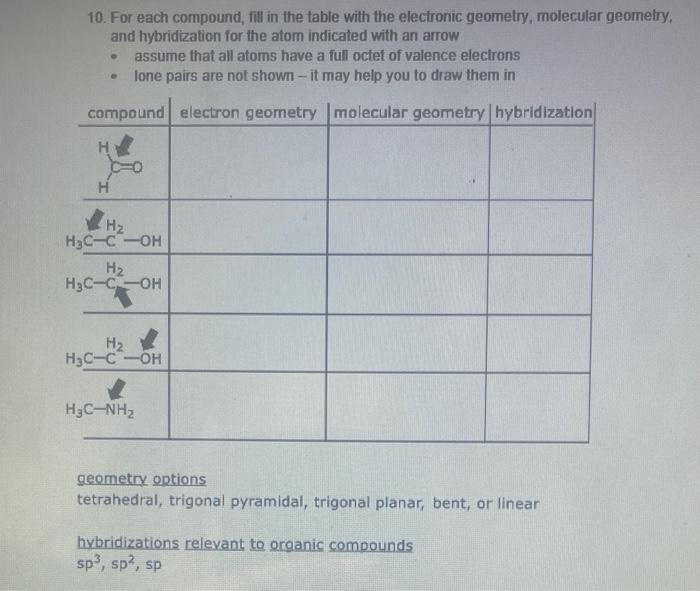
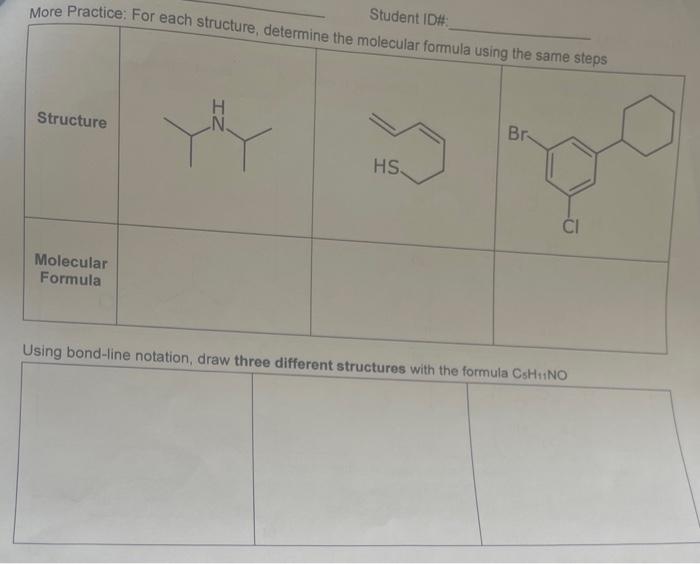
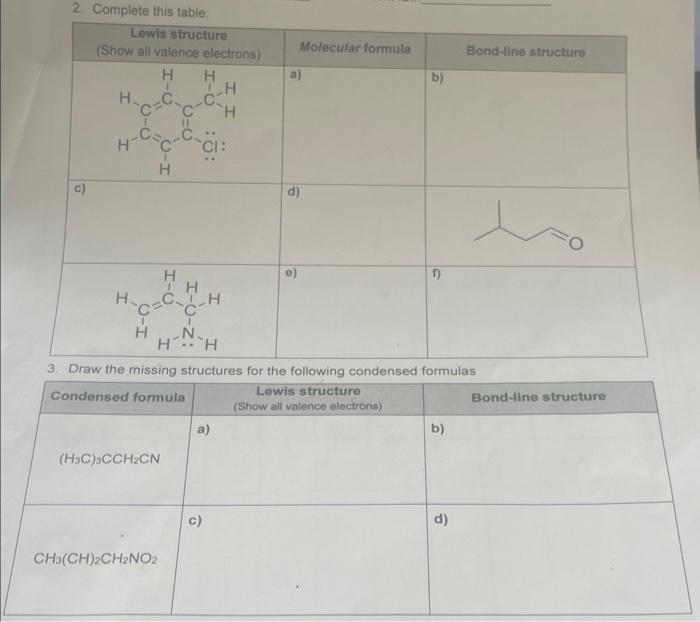
10. For each compound, fil in the table with the electronic geometry, molecular geomelry, and hybridization for the atom indicated with an arrow - assume that all atoms have a full octet of valence elecirons - lone pairs are not shown - it may help you to draw them in geometry options tetrahedral, trigonal pyramidal, trigonal planar, bent, or linear hybridizations relevant to organic compounds sp3,sp2,sp More Practice: For each structure, determine the molecular formula using the same steps Structure Molecular Formula Using bond-line notation, draw three different structures with the formula CsH1NO 2. Complete this table: 3. Draw the missing structures for the following condensed formulas \begin{tabular}{|l|l|l|} \hline Condensed formula & Lewis structure (Show all valence electrons) & Bond-line structure \\ \hline(H3C3CCH2CN & a) & b) \\ \hline CH(CH)2CH2NO2 & c) & d) \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


