Question: #10 keeps saying thats the wrong answer for the last question in grams Be sure to answer all parts. Metal hydrides react with water to
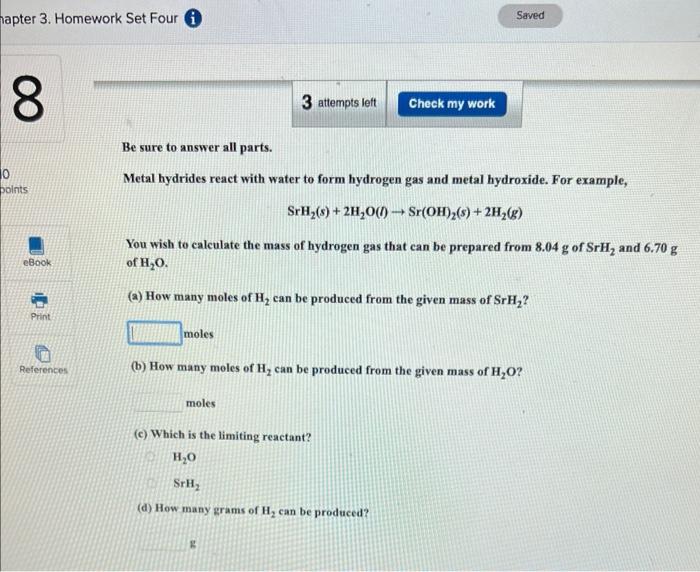
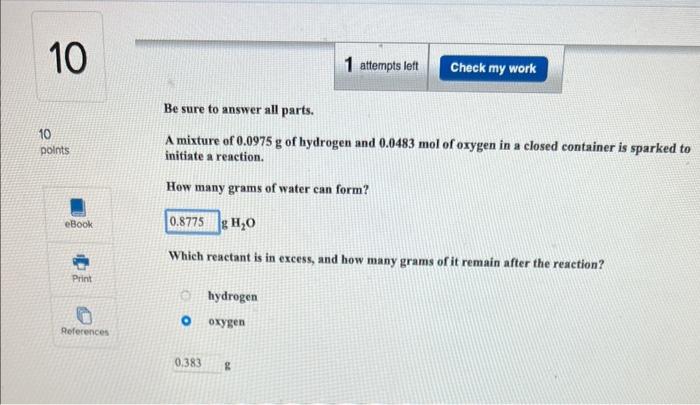
Be sure to answer all parts. Metal hydrides react with water to form hydrogen gas and metal hydroxide. For example, SrH2(s)+2H2O(l)Sr(OH)2(s)+2H2(g) You wish to calculate the mass of hydrogen gas that can be prepared from 8.04g of SrH2 and 6.70g of H2O. (a) How many moles of H2 can be produced from the given mass of SrH2 ? moles (b) How many moles of H2 can be produced from the given mass of H2O ? moles (c) Which is the limiting reactant? H2O SrH2 (d) How many grams of H2 can be produced? g Be sure to answer all parts. A mixture of 0.0975g of hydrogen and 0.0483mol of oxygen in a closed container is sparked to initiate a reaction. Hew many grams of water can form? ( H2O Which reactant is in excess, and how many grams of it remain after the reaction? hydrogen oxygen Be sure to answer all parts. Metal hydrides react with water to form hydrogen gas and metal hydroxide. For example, SrH2(s)+2H2O(l)Sr(OH)2(s)+2H2(g) You wish to calculate the mass of hydrogen gas that can be prepared from 8.04g of SrH2 and 6.70g of H2O. (a) How many moles of H2 can be produced from the given mass of SrH2 ? moles (b) How many moles of H2 can be produced from the given mass of H2O ? moles (c) Which is the limiting reactant? H2O SrH2 (d) How many grams of H2 can be produced? g Be sure to answer all parts. A mixture of 0.0975g of hydrogen and 0.0483mol of oxygen in a closed container is sparked to initiate a reaction. Hew many grams of water can form? ( H2O Which reactant is in excess, and how many grams of it remain after the reaction? hydrogen oxygen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


