Question: (10 points) In quantum mechanics, the state is a wavefunction dened as I'. For an electron bound in a hydrogen atom, these wavefunctions are well
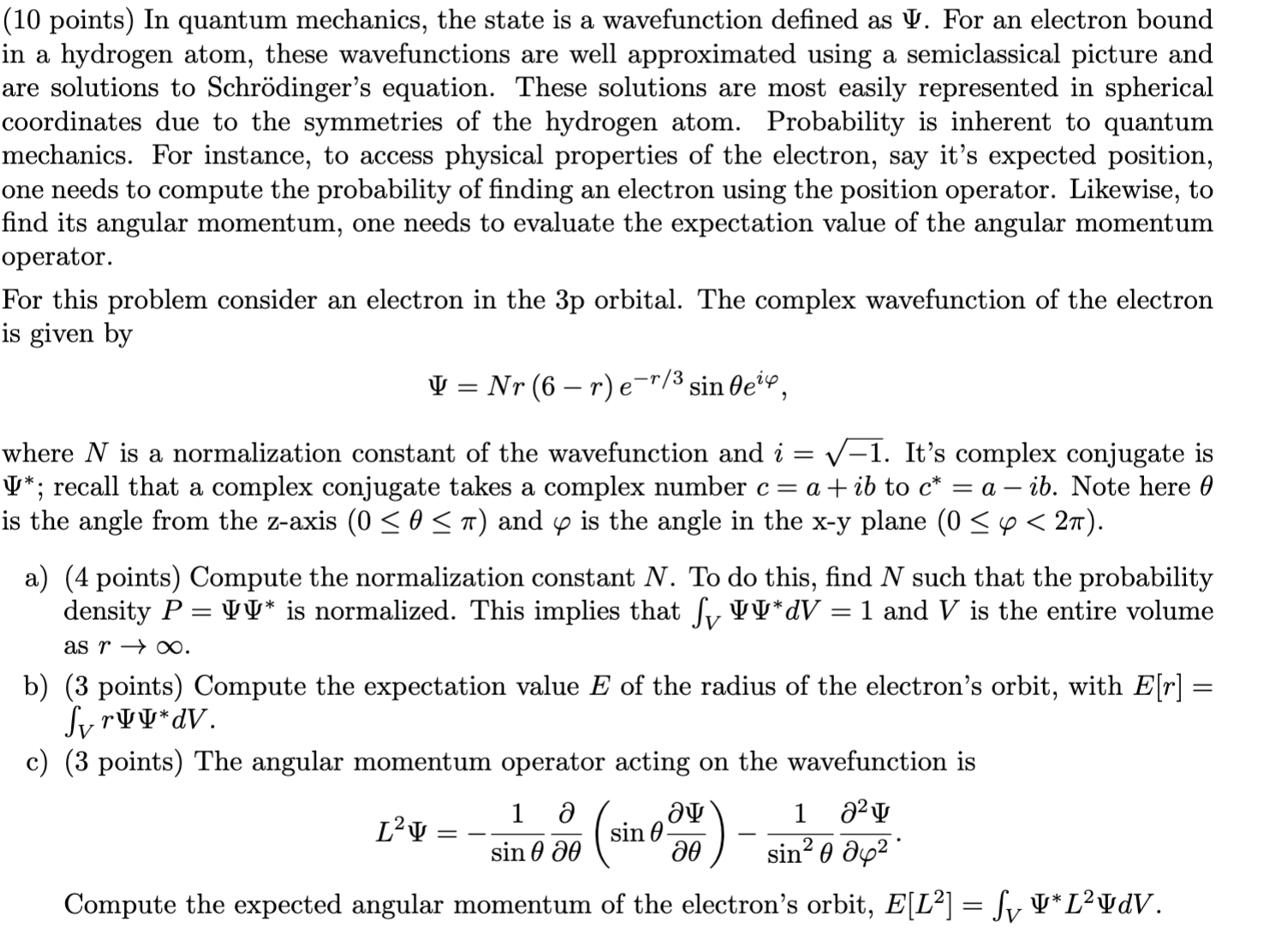
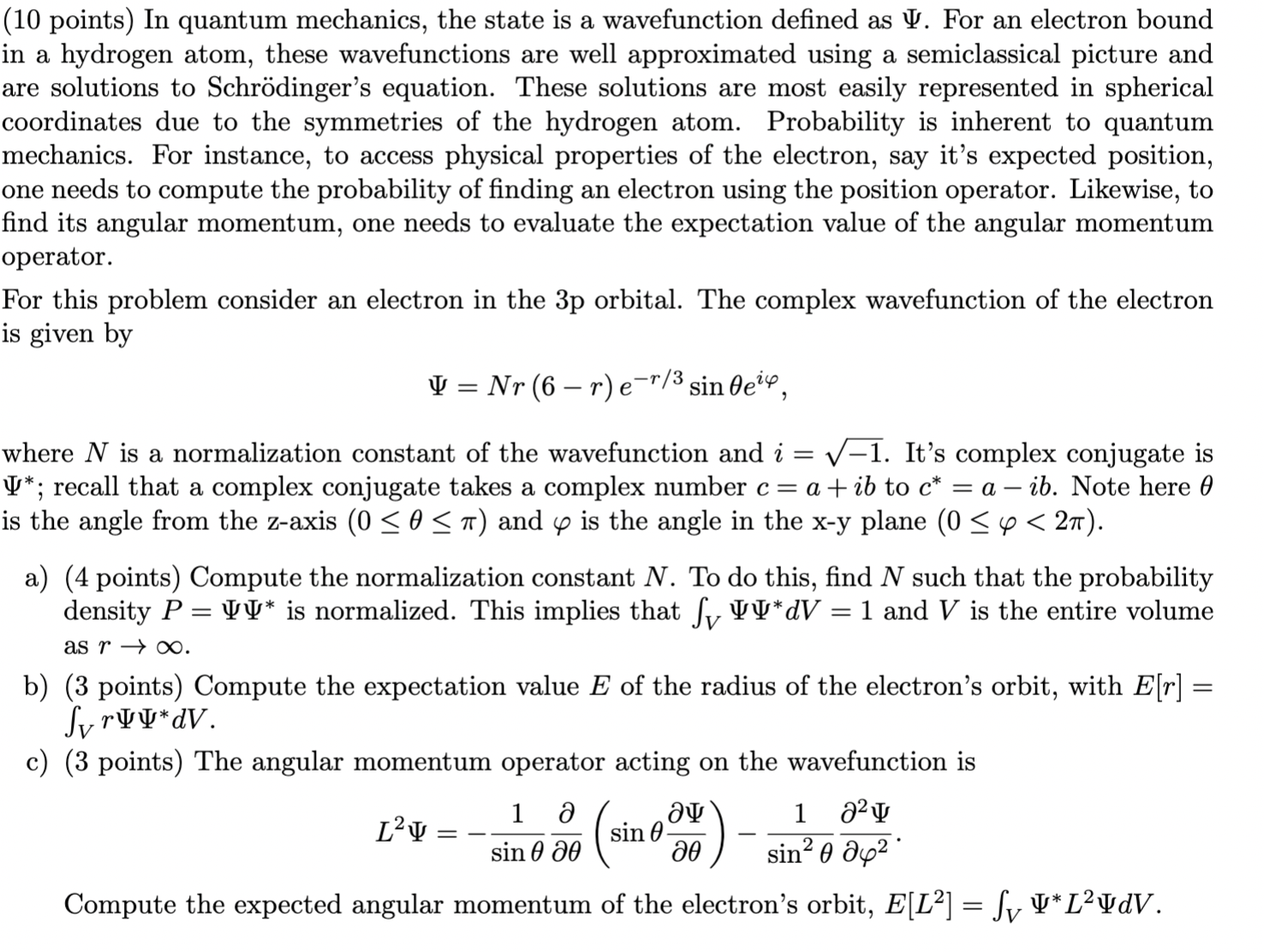
(10 points) In quantum mechanics, the state is a wavefunction dened as \\I'. For an electron bound in a hydrogen atom, these wavefunctions are well approximated using a semiclassical picture and are solutions to Schrdinger's equation. These solutions are most easily represented in spherical coordinates due to the symmetries of the hydrogen atom. Probability is inherent to quantum mechanics. For instance, to access physical properties of the electron, say it's expected position, one needs to compute the probability of nding an electron using the position operator. Likewise, to nd its angular momentum, one needs to evaluate the expectation value of the angular momentum operator. For this problem consider an electron in the 3p orbital. The complex wavefunction of the electron is given by II! 2 NT (6 r) fir/3 sinBei'P, Where N is a normalization constant of the wavefunction and 2' = \\/1. It's complex conjugate is '1'\"; recall that a complex conjugate takes a complex number 0 = a. + ib to c* = a 73!). Note here 9 is the angle from the z-axis (0 S 0 5 11') and (p is the angle in the xy plane (0 5 cp
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


