Question: 10) Write the total differential for entropy, S as a function of temperature, T and pressure, P. Given that, (TS)r=TCp=2T5Rand(PS)T=(TV)p=PR for 1 mole of ideal
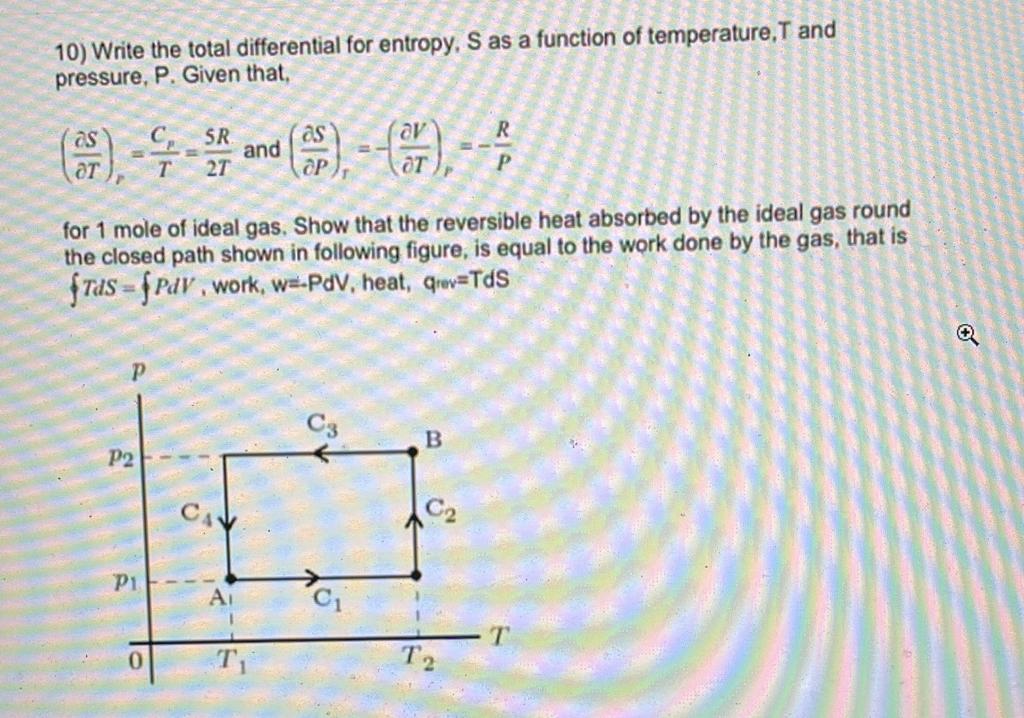
10) Write the total differential for entropy, S as a function of temperature, T and pressure, P. Given that, (TS)r=TCp=2T5Rand(PS)T=(TV)p=PR for 1 mole of ideal gas. Show that the reversible heat absorbed by the ideal gas round the closed path shown in following figure, is equal to the work done by the gas, that is TdS=PdV, work, we.PdV, heat, qrav =TdS
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


