Question: 1003 has been transferred to the block. Experiment Complete Click Reset for a new experiment. Specific heat capacity is a measure of the quantity of
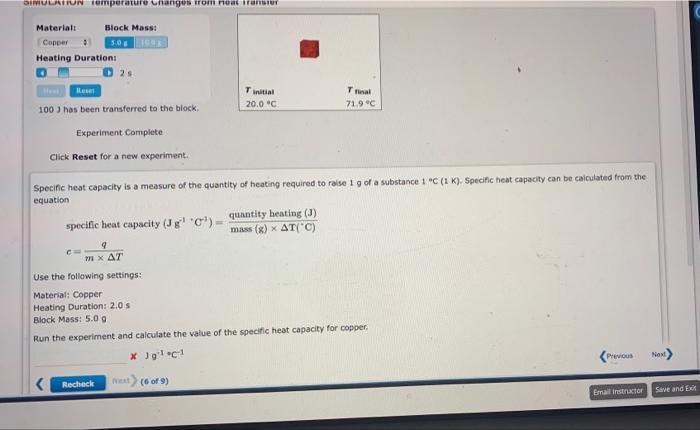
1003 has been transferred to the block. Experiment Complete Click Reset for a new experiment. Specific heat capacity is a measure of the quantity of heating required to raise 1g of a substance 1 ac ( 1K ). Specific heat capacity can be caiculated from the equation specific heat capacity (J1+C1)=mass(g)T(+C)quantityheating(J) c=mTq Use the following settings: Material: Copper Heating Duration: 2.0 s Block Mass: 5.0g Run the experiment and calculate the value of the specific heat capacity for copper. Jg1C1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


