Question: 10.1 Plot the equilibrium data on the following coordinate systems: (a) triangular; (b)x and y against weight fraction B;(c)x against y. 10.2 Compute the selectivity

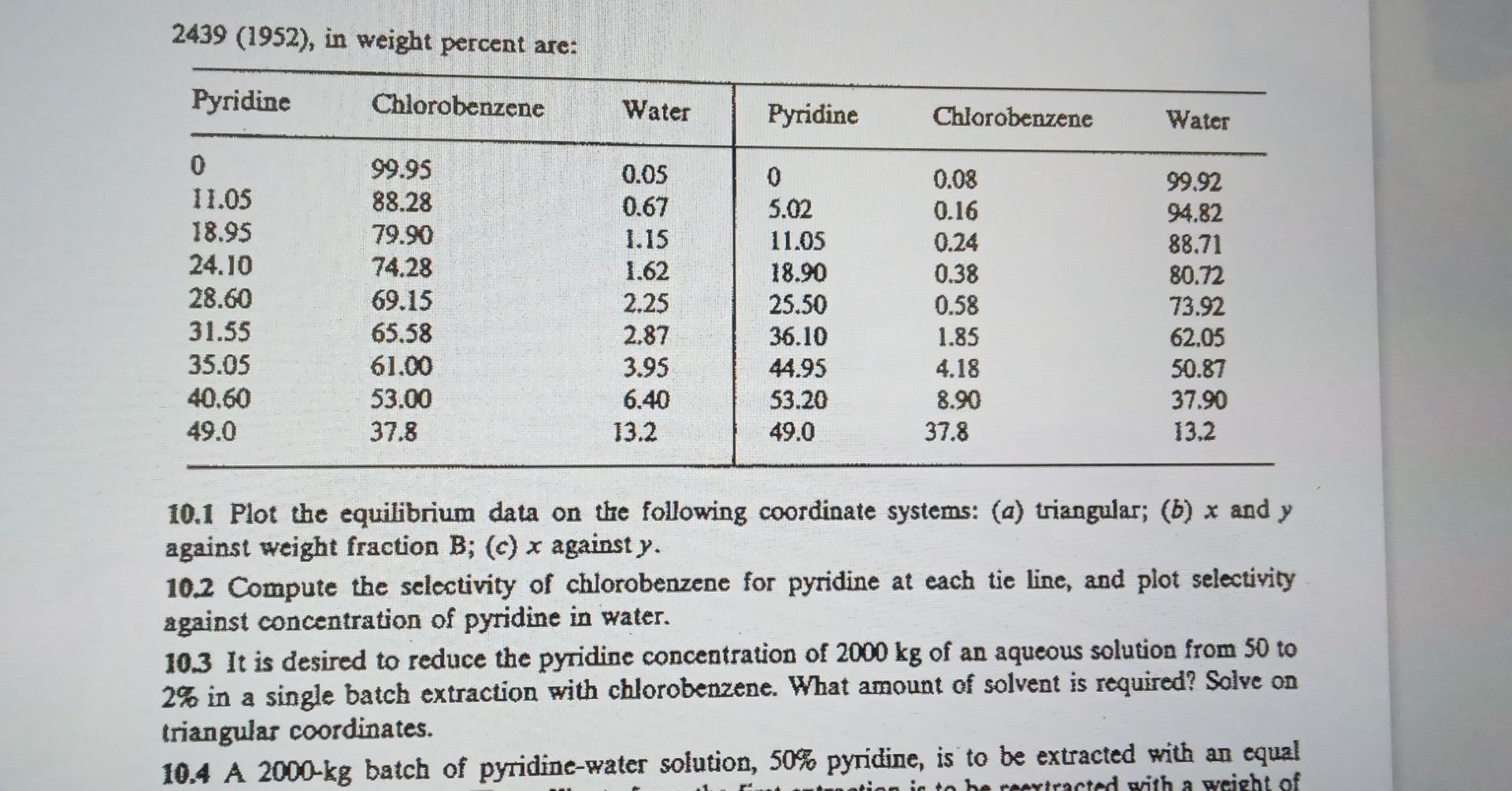
10.1 Plot the equilibrium data on the following coordinate systems: (a) triangular; (b)x and y against weight fraction B;(c)x against y. 10.2 Compute the selectivity of chlorobenzene for pyridine at each tie line, and plot selectivity against concentration of pyridine in water. 10.3 It is desired to reduce the pyridine concentration of 2000kg of an aqueous solution from 50 to 2% in a single batch extraction with chlorobenzene. What amount of solvent is required? Solve on triangular coordinates. 2439(1952), in weight percent are: 10.1 Plot the equilibrium data on the following coordinate systems: (a) triangular; (b)x and y against weight fraction B;(c)x against y. 10.2 Compute the selectivity of chlorobenzene for pyridine at each tie line, and plot selectivity against concentration of pyridine in water. 10.3 It is desired to reduce the pyridine concentration of 2000kg of an aqueous solution from 50 to 2% in a single batch extraction with chlorobenzene. What amount of solvent is required? Solve on triangular coordinates. 10.4 A 2000kg batch of pyridine-water solution, 50% pyridine, is to be extracted with an equal 10.1 Plot the equilibrium data on the following coordinate systems: (a) triangular; (b)x and y against weight fraction B;(c)x against y. 10.2 Compute the selectivity of chlorobenzene for pyridine at each tie line, and plot selectivity against concentration of pyridine in water. 10.3 It is desired to reduce the pyridine concentration of 2000kg of an aqueous solution from 50 to 2% in a single batch extraction with chlorobenzene. What amount of solvent is required? Solve on triangular coordinates. 2439(1952), in weight percent are: 10.1 Plot the equilibrium data on the following coordinate systems: (a) triangular; (b)x and y against weight fraction B;(c)x against y. 10.2 Compute the selectivity of chlorobenzene for pyridine at each tie line, and plot selectivity against concentration of pyridine in water. 10.3 It is desired to reduce the pyridine concentration of 2000kg of an aqueous solution from 50 to 2% in a single batch extraction with chlorobenzene. What amount of solvent is required? Solve on triangular coordinates. 10.4 A 2000kg batch of pyridine-water solution, 50% pyridine, is to be extracted with an equal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


