Question: 11. Consider the Ka values for acids X and Y given below. Acid X is than acid Y, because it has a value of Ka.
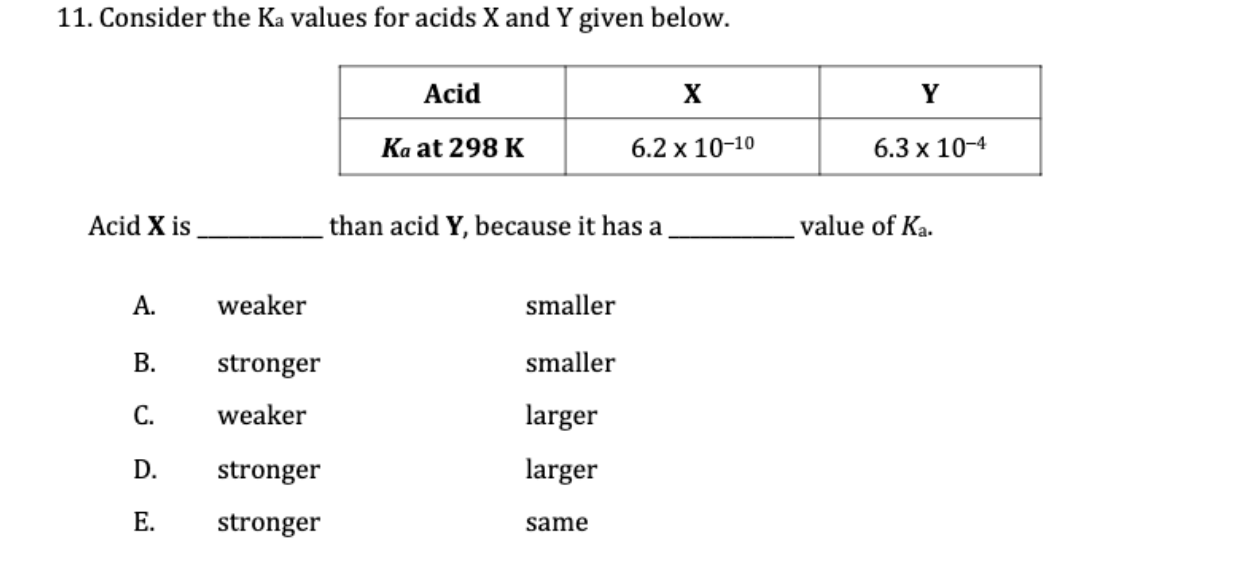
11. Consider the Ka values for acids X and Y given below. Acid X is than acid Y, because it has a value of Ka. A. weaker smaller B. stronger smaller C. weaker larger D. stronger larger E. stronger same
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


