Question: .11 Gas A diffuses through two immiscible liquids contained in a capillary tube as shown in Figure 3-11. The concentration of A at the bottom
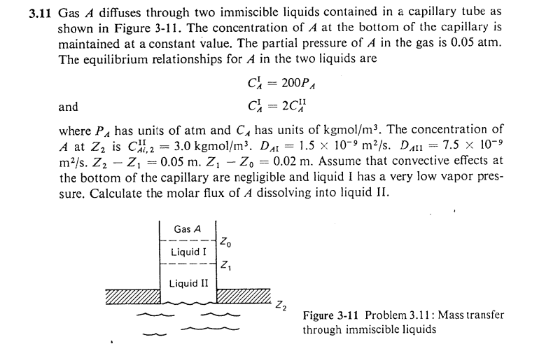
.11 Gas A diffuses through two immiscible liquids contained in a capillary tube as shown in Figure 3-11. The concentration of A at the bottom of the capillary is maintained at a constant value. The partial pressure of A in the gas is 0.05atm. The equilibrium relationships for A in the two liquids are CAI=200PACAI=2CAII and where PA has units of atm and CA has units of kgmol/m3. The concentration of A at Z2 is CAi,21I=3.0kgmol/m3.DAI=1.5109m2/s.DAH=7.5109 m2/s.Z2Z1=0.05m.Z1Z0=0.02m. Assume that convective effects at the bottom of the capillary are negligible and liquid I has a very low vapor pressure. Calculate the molar flux of A dissolving into liquid II. Figure 3-11 Problem 3.11: Mass transfer through immiscible liquids
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


