Question: 11) In front of each step below, write the name of each type of energy. Na(s)Na(g)Cl2(g)2Cl(g)Na(g)Na+(g)+eCl(g)+eCl(g)Na(s)+21Cl2(g)NaCl(s)H=109kJH=243kJH=496kJH=()349kJH=()411kJ Use the information above to calculate the H iatilice
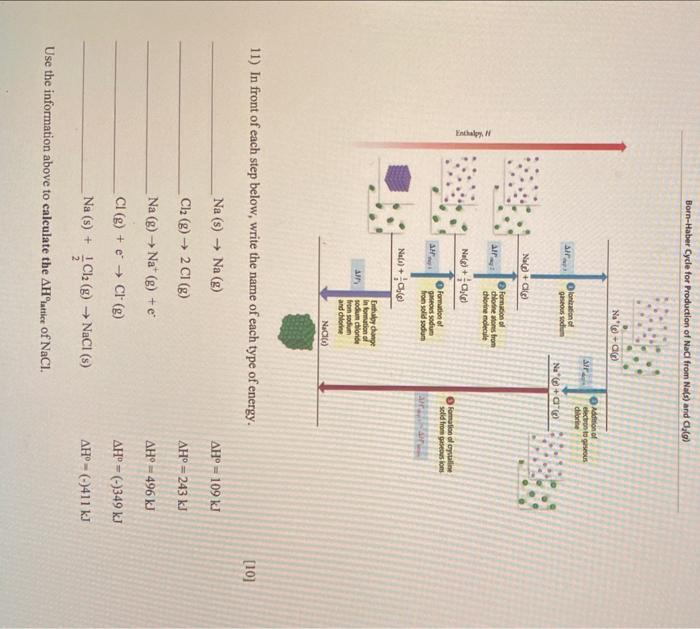
11) In front of each step below, write the name of each type of energy. Na(s)Na(g)Cl2(g)2Cl(g)Na(g)Na+(g)+eCl(g)+eCl(g)Na(s)+21Cl2(g)NaCl(s)H=109kJH=243kJH=496kJH=()349kJH=()411kJ Use the information above to calculate the H iatilice of NaCl
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


