Question: -11 View Policies Current Attempt in Progress Consider a 25.0 mol sample of gas consisting of the following components by weight percent Component Weight Percent
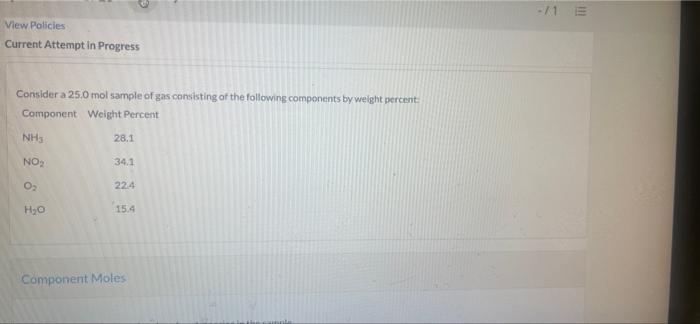
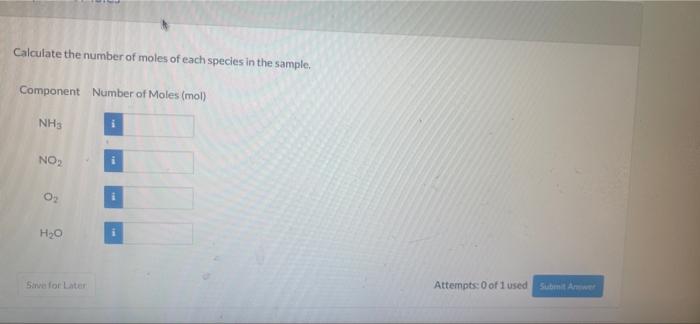
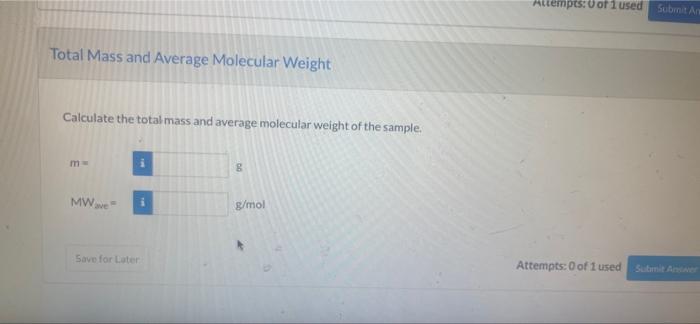
-11 View Policies Current Attempt in Progress Consider a 25.0 mol sample of gas consisting of the following components by weight percent Component Weight Percent NH 28.1 NO 34.1 O 224 H, 15.4 Component Moles Calculate the number of moles of each species in the sample. Component Number of Moles (mol) NHS NO2 i O2 H2O Sirve for Later Attempts: 0 of 1 used Submit Awe Attempts: 0 of 1 used Submit a Total Mass and Average Molecular Weight Calculate the total mass and average molecular weight of the sample. m 00 MWave g/mol Savo for Later Attempts: 0 of 1 used Sutonite
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


