Question: Use the two-point version of the Clausius-Clapeyron equation. The vapor pressure of liquid bismuth is 400. mm Hg at 1.72x10 K. Assuming that AHvap
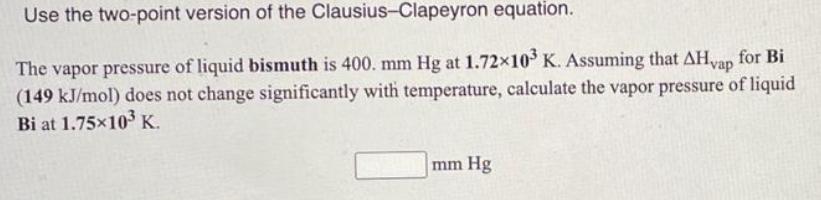
Use the two-point version of the Clausius-Clapeyron equation. The vapor pressure of liquid bismuth is 400. mm Hg at 1.72x10 K. Assuming that AHvap for Bi (149 kJ/mol) does not change significantly with temperature, calculate the vapor pressure of liquid Bi at 1.7510 K. mm Hg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


