Question: 11.9. For a ternary solution at constant T and P, the composition dependence of molar property M is given by: M = x1 M1 +
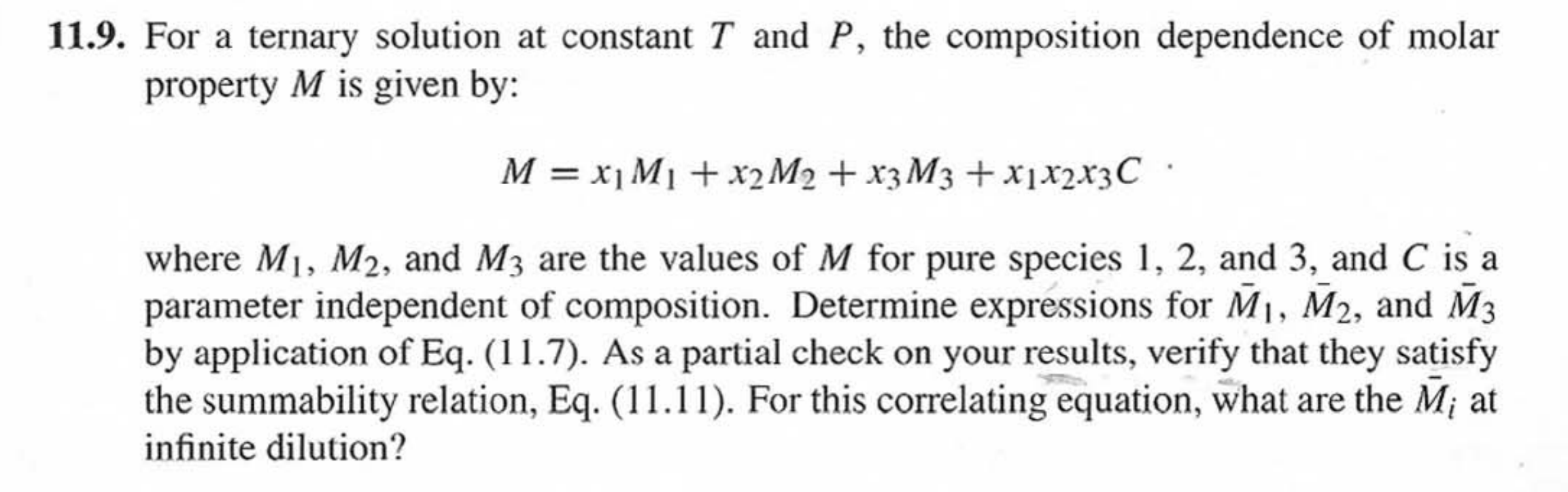
11.9. For a ternary solution at constant T and P, the composition dependence of molar property M is given by: M = x1 M1 + x2 M2 + x3M3 + x[x2x3C. where M1, M2, and M3 are the values of M for pure species 1, 2, and 3, and C is a parameter independent of composition. Determine expressions for M, M2, and M3 by application of Eq. (11.7). As a partial check on your results, verify that they satisfy the summability relation, Eq. (11.11). For this correlating equation, what are the M; at infinite dilution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


