Question: 12 0 points earned Carbon monoxide has how many pi and sigma bonds? 1 pi and 1 sigma 1 pi and 2 sigma O2 pi
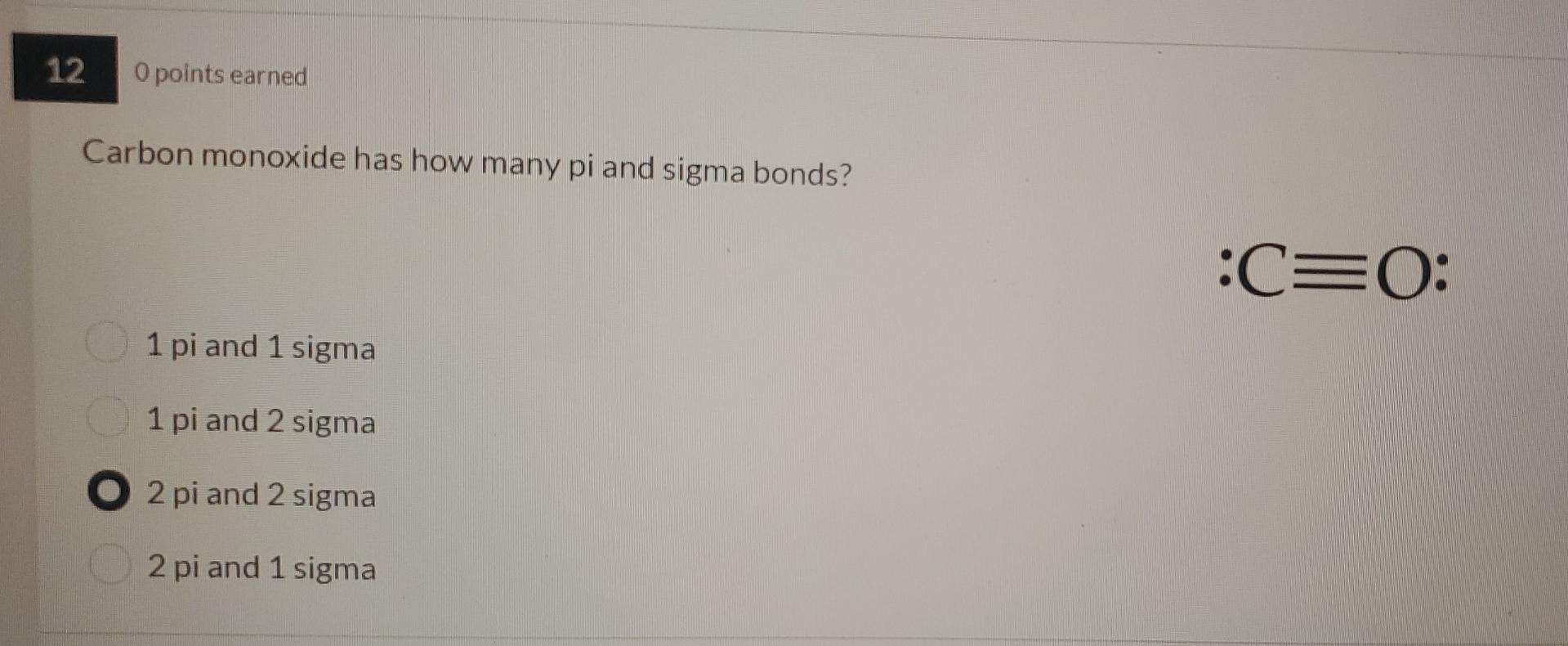

12 0 points earned Carbon monoxide has how many pi and sigma bonds? 1 pi and 1 sigma 1 pi and 2 sigma O2 pi and 2 sigma 2 pi and 1 sigma :C=0: 17 Opoints earned According to MO Theory, overlap of two s atomic orbitals produces two bonding molecular orbitals and two antibonding molecular orbitals one bonding molecular orbital and one antibonding molecular orbital two bonding orbitals one bonding orbital and one hybrid orbital
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


