Question: 12: Methane gas (CH) is diffusing at constant rate through a layer of stagnant air 1mm thick. Conditions are fixed so that the mole fraction
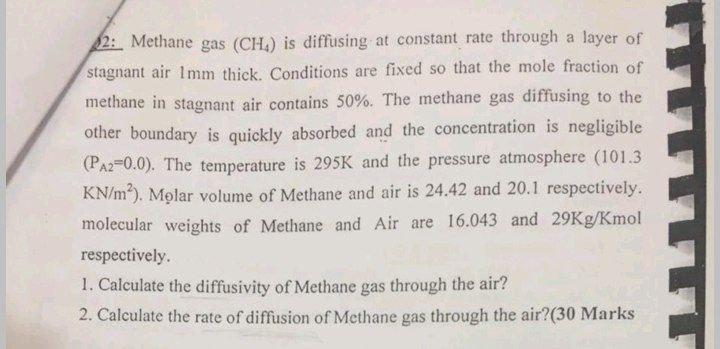
12: Methane gas (CH) is diffusing at constant rate through a layer of stagnant air 1mm thick. Conditions are fixed so that the mole fraction of methane in stagnant air contains 50%. The methane gas diffusing to the other boundary is quickly absorbed and the concentration is negligible (PA2=0.0). The temperature is 295K and the pressure atmosphere (101.3 KN/m?). Molar volume of Methane and air is 24.42 and 20.1 respectively. molecular weights of Methane and Air are 16.043 and 29Kg/Kmol respectively. 1. Calculate the diffusivity of Methane gas through the air? 2. Calculate the rate of diffusion of Methane gas through the air?(30 Marks
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


