Question: 12) Nitrogen case: The N-H stretching absorption is less sensitive to hydrogen bonding than are OH absorptions. In the gas phase and in dilute CCl4
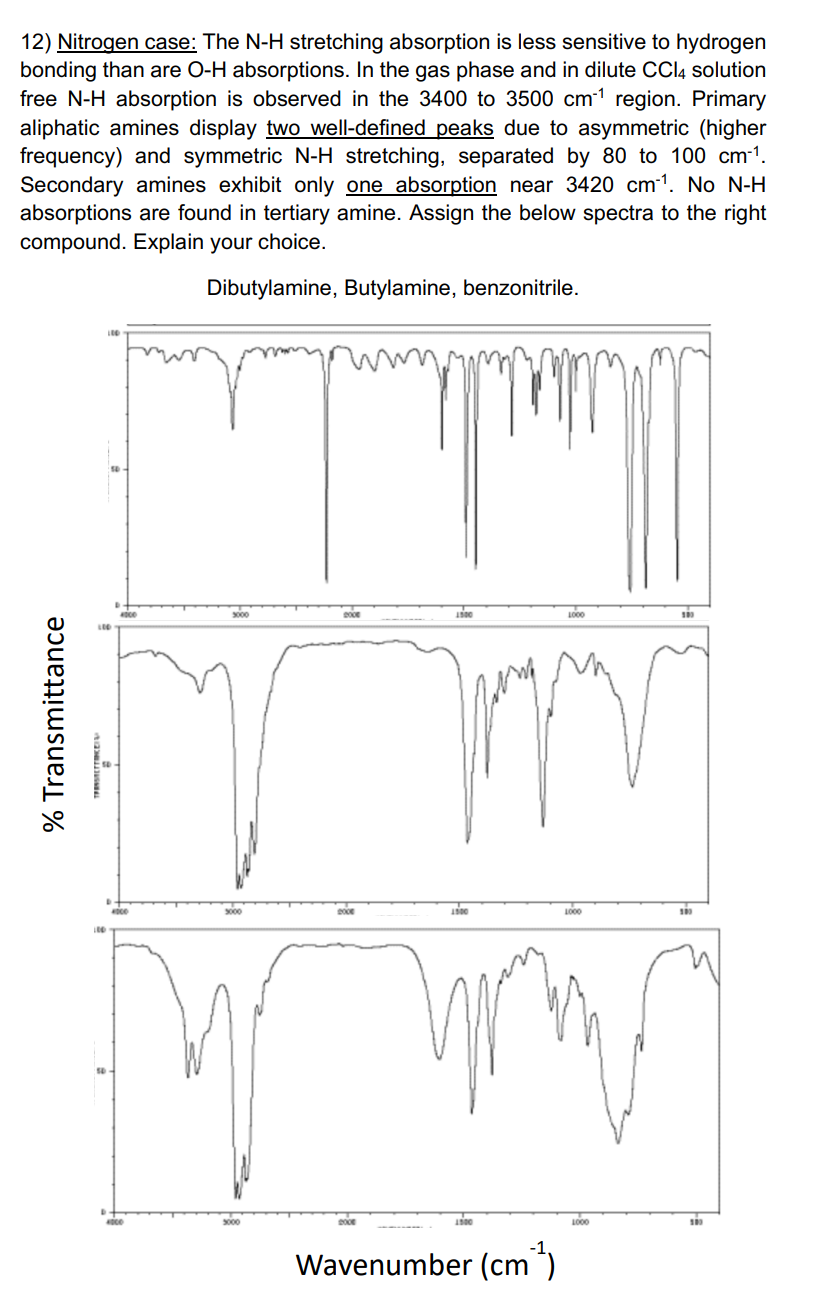
12) Nitrogen case: The N-H stretching absorption is less sensitive to hydrogen bonding than are OH absorptions. In the gas phase and in dilute CCl4 solution free NH absorption is observed in the 3400 to 3500cm1 region. Primary aliphatic amines display two well-defined peaks due to asymmetric (higher frequency) and symmetric NH stretching, separated by 80 to 100cm1. Secondary amines exhibit only one absorption near 3420cm1. No NH absorptions are found in tertiary amine. Assign the below spectra to the right compound. Explain your choice. Dibutylamine, Butylamine, benzonitrile
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


