Question: 1,2,3 pleasee i need to pass this class An aqueous solution is 3.50% by mass potassium bromide, KBr, and has a density of 1.02 g/mL.
1,2,3 pleasee i need to pass this class 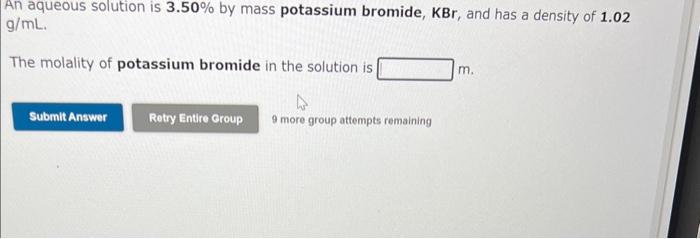

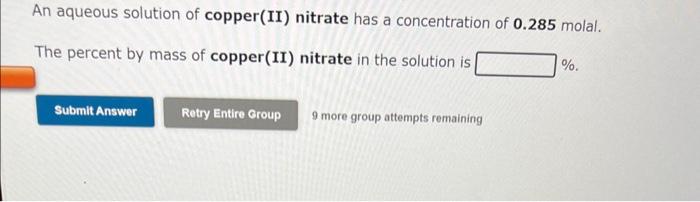
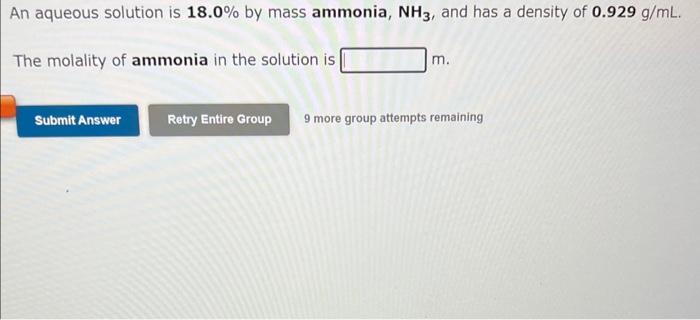
An aqueous solution is 3.50% by mass potassium bromide, KBr, and has a density of 1.02 g/mL. The molality of potassium bromide in the solution is m. 9 more group attempts remaining An aqueous solution of copper(II) nitrate has a concentration of 0.285 molal. The percent by mass of copper(II) nitrate in the solution is % An aqueous solution is 18.0% by mass ammonia, NH3, and has a density of 0.929g/mL. The molality of ammonia in the solution is m. 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


