Question: 13. How many grams of solid PbCl2 form when 15.3g of NaCl(aq) reacts with 60.8g of Pb(NO3)2(aq) according to the balanced chemical equation shown below?
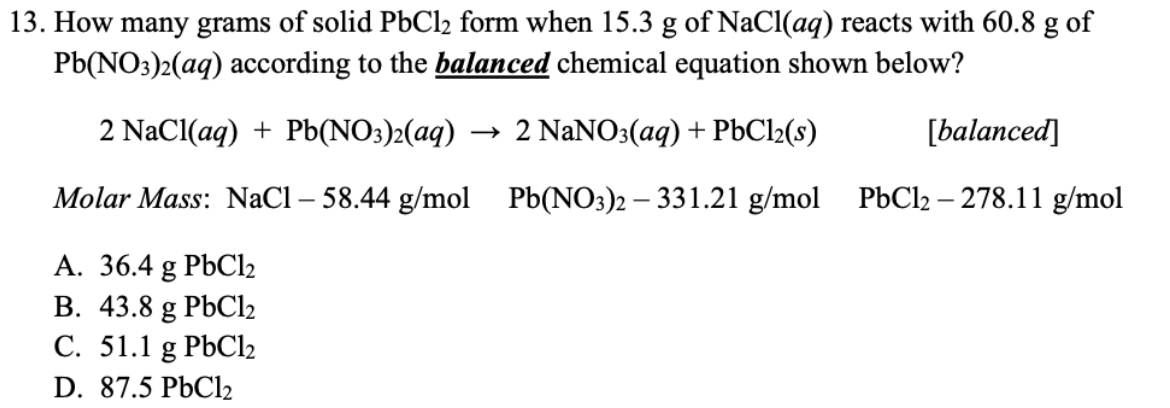
13. How many grams of solid PbCl2 form when 15.3g of NaCl(aq) reacts with 60.8g of Pb(NO3)2(aq) according to the balanced chemical equation shown below? 2NaCl(aq)+Pb(NO3)2(aq)2NaNO3(aq)+PbCl2(s)[balanced] Molar Mass: NaCl58.44g/molPb(NO3)2331.21g/molPbCl2278.11g/mol A. 36.4gPbCl2 B. 43.8gPbCl2 C. 51.1gPbCl2 D. 87.5PbCl2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


