Question: 13.43 Ammonium nitrite, NH4NO2, decomposes in solution, as shown here. NH4NO2(aq)N2(g)+2H2O(l) The concentration of NH4+ion at the beginning of an experiment was 0.500M. After 3.00
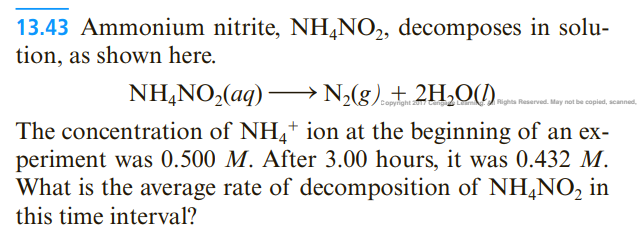
13.43 Ammonium nitrite, NH4NO2, decomposes in solution, as shown here. NH4NO2(aq)N2(g)+2H2O(l) The concentration of NH4+ion at the beginning of an experiment was 0.500M. After 3.00 hours, it was 0.432M. What is the average rate of decomposition of NH4NO2 in this time interval
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


