Question: 15. Determine whether the following reaction involves carbon oxidation or reduction. Please show the oxidation numbers of each carbon. 2CH2O(g)+O2(g)2CH2O2(g) 16. In general, oxidation of
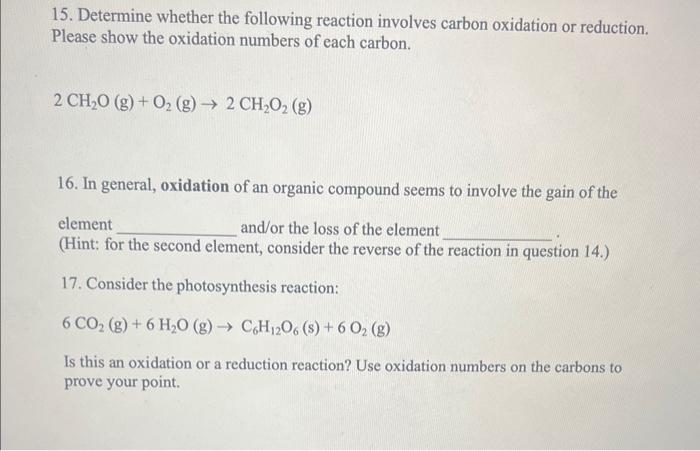

15. Determine whether the following reaction involves carbon oxidation or reduction. Please show the oxidation numbers of each carbon. 2CH2O(g)+O2(g)2CH2O2(g) 16. In general, oxidation of an organic compound seems to involve the gain of the element and/or the loss of the element (Hint: for the second element, consider the reverse of the reaction in question 14.) 17. Consider the photosynthesis reaction: 6CO2(g)+6H2O(g)C6H12O6(s)+6O2(g) Is this an oxidation or a reduction reaction? Use oxidation numbers on the carbons to prove your point. 18. In general, reduction of an organic compound seems to involve the gain of the element and/or the loss of the element
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


