Question: 16) Which compound in each pair has the higher boiling point? Explain. a. (CH3)3CCH2CH3 or CH3(CH2)4CH3 b. CH3CH2CH2OH or CH3CH2COOH c. 1-butanol or 1-bromobutane d.
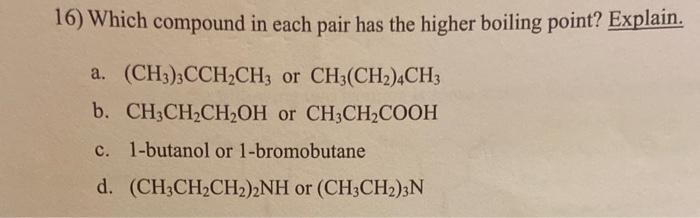
16) Which compound in each pair has the higher boiling point? Explain. a. (CH3)3CCH2CH3 or CH3(CH2)4CH3 b. CH3CH2CH2OH or CH3CH2COOH c. 1-butanol or 1-bromobutane d. (CH3CH2CH2)2NH or (CH3CH2)3N
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


