Question: 16. Which process below has been described correctly for a temperature above 274K ? a. H2O(I)H^2O(s) is exothermic and spontaneous. b. H2O(I)H2O(s) is endothermic and
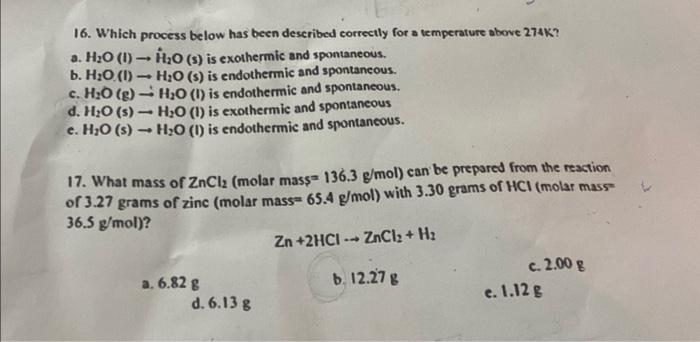
16. Which process below has been described correctly for a temperature above 274K ? a. H2O(I)H^2O(s) is exothermic and spontaneous. b. H2O(I)H2O(s) is endothermic and spontaneous. c. H2O(g)H2O(I) is endothermic and spontaneous. d. H2O(s)H2O (I) is exothermic and spontaneous e. H2O(s)H2O(I) is endothermic and spontaneous. 17. What mass of ZnCl2 (molar mass 136.3g/mol ) can be prepared from the reaction of 3.27 grams of zinc (molar mass =65.4g/mol ) with 3.30 grams of HCl (molar mass 36.5g/mol) ? Zn+2HClZnCl2+H2 a. 6.82g b. 12.27g c. 2.008 d. 6.13g e. 1.12g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


