Question: 17. Complete the Ice table using the information that the final concentration of CoCl42 is 0.003204M. Make sure to use the initial concentrations you calculated
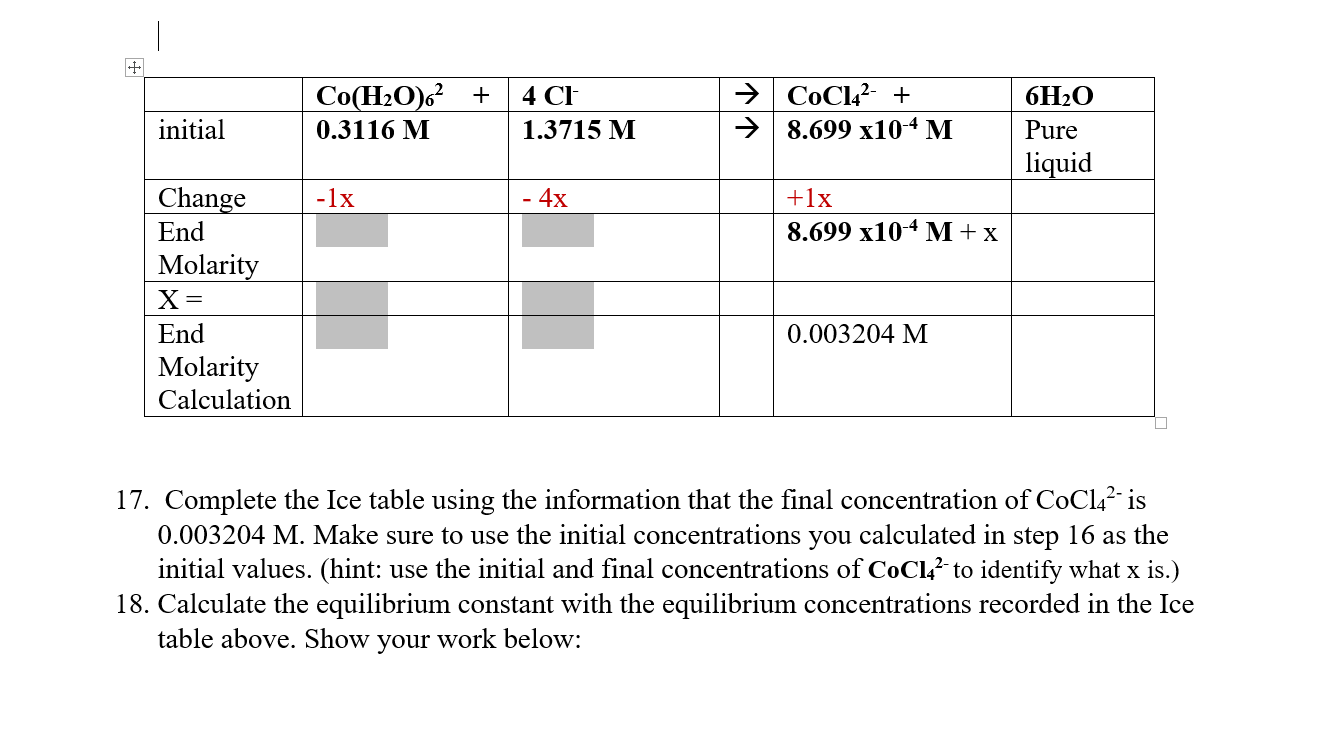
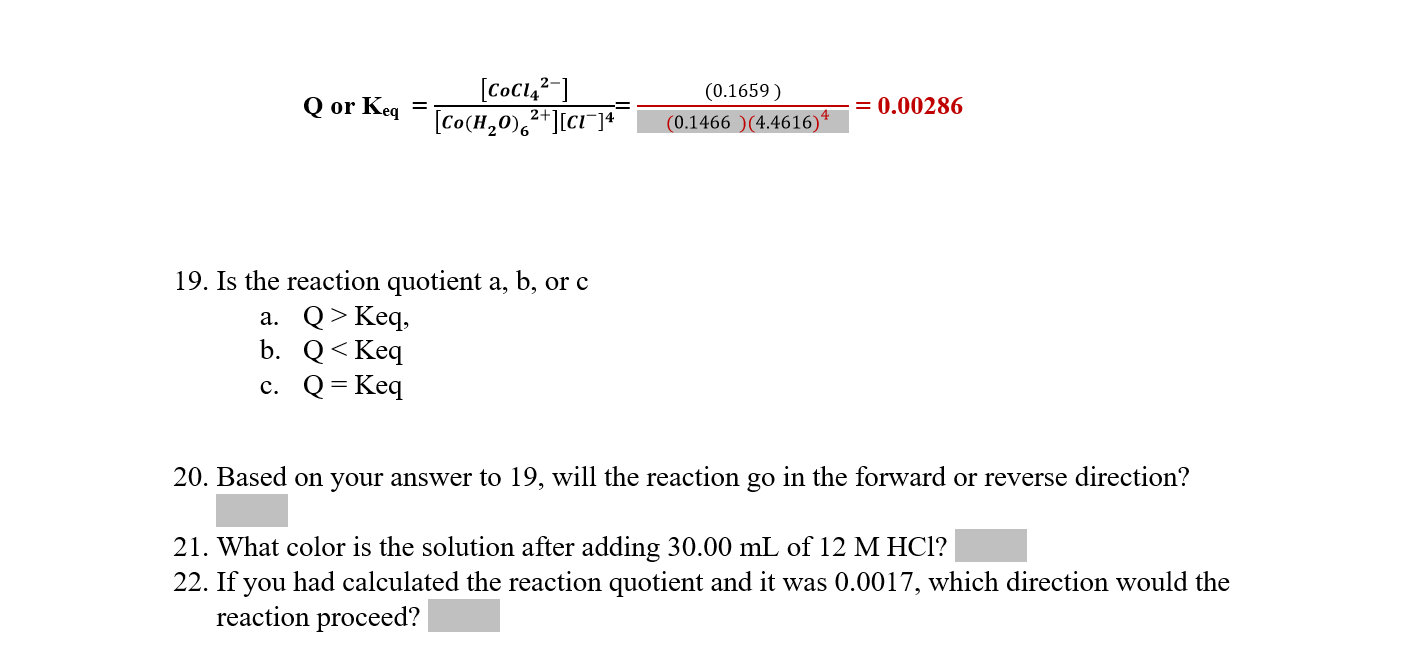
17. Complete the Ice table using the information that the final concentration of CoCl42 is 0.003204M. Make sure to use the initial concentrations you calculated in step 16 as the initial values. (hint: use the initial and final concentrations of CoCl42 to identify what x is.) 18. Calculate the equilibrium constant with the equilibrium concentrations recorded in the Ice table above. Show your work below: 19. Is the reaction quotient a,b, or c a. Q>Keq, b. Q
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


