Question: 18. Given the following data for boron trichloride at 25 C, calculate S for BCls(D. AHP BCl5() =-427.2 kJ/mol; AHP BCh(g) =-403.8 kJ/mol; S
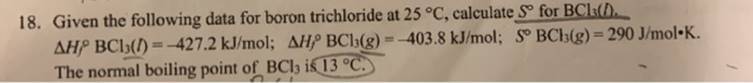
18. Given the following data for boron trichloride at 25 C, calculate S for BCls(D. AHP BCl5() =-427.2 kJ/mol; AHP BCh(g) =-403.8 kJ/mol; S BCb(g)= 290 J/mol-K. The normal boiling point of BCl ik 13 C. %3D !3!
Step by Step Solution
★★★★★
3.46 Rating (156 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Gimen A ket bas A 4272 KJmol a Jmolk 4038 KJ mo 290 J lamolk No... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


