Question: 1a. Why does diethyl ether have a low boiling point, but a high solubility in water? Your answer should relate to Intermolecular forces. 1b. Why
1a. Why does diethyl ether have a low boiling point, but a high solubility in water? Your answer should relate to Intermolecular forces.
1b. Why does Dichloromethane have a lower boiling point than Chloroform? Your answer should relate to Intermolecular forces.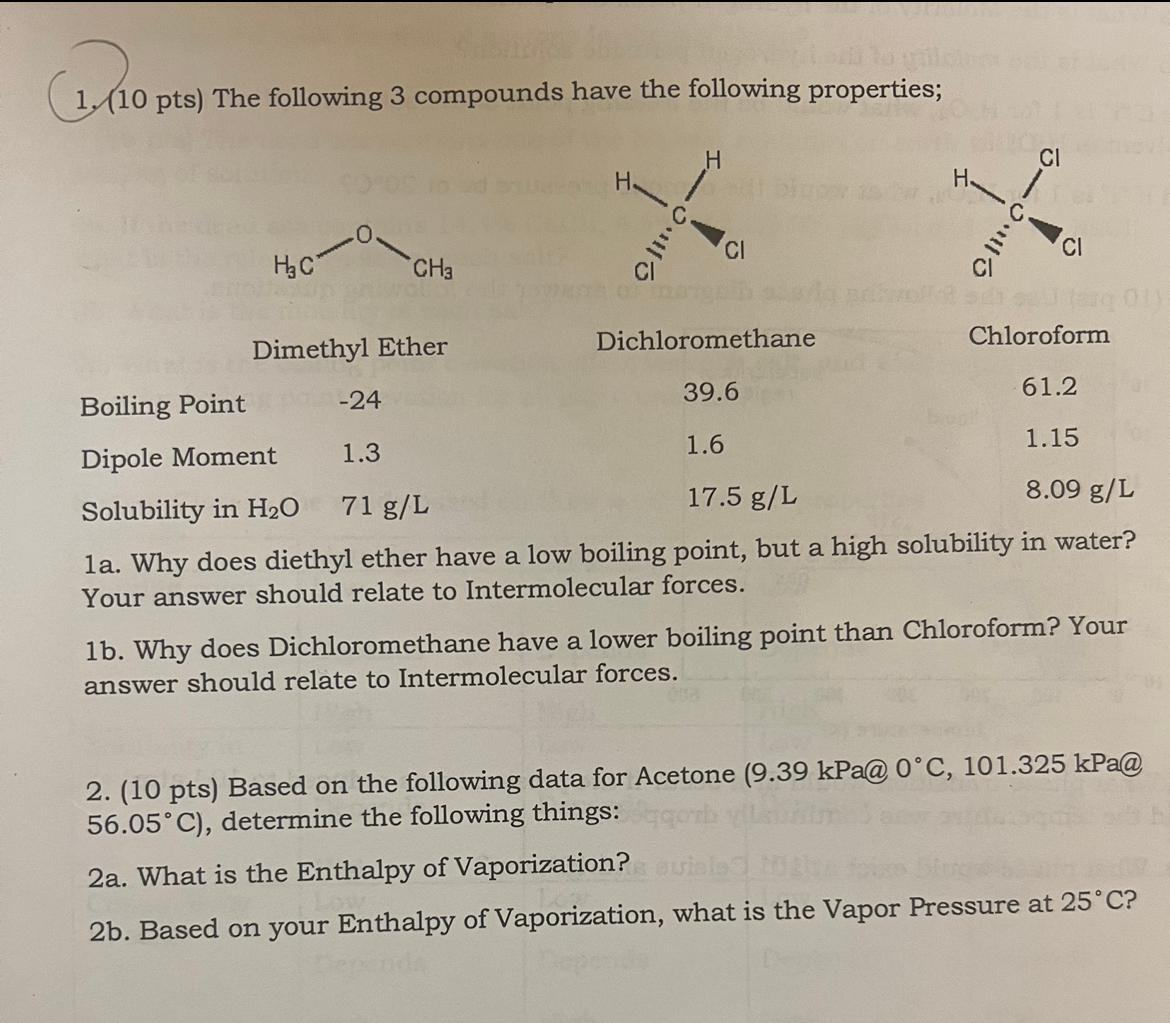
1. (10 pts) The following 3 compounds have the following properties; 1a. Why does diethyl ether have a low boiling point, but a high solubility in water? Your answer should relate to Intermolecular forces. 1b. Why does Dichloromethane have a lower boiling point than Chloroform? Your answer should relate to Intermolecular forces. 2. (10 pts) Based on the following data for Acetone 9.39kPa@0C,101.325kPa@ 56.05C ), determine the following things: 2a. What is the Enthalpy of Vaporization? 2b. Based on your Enthalpy of Vaporization, what is the Vapor Pressure at 25C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


