Question: /2 1. What was the evidence that the cathode rays were particles with charge and mass? Answer:7. In a hydrogen atom, the electron jumps from


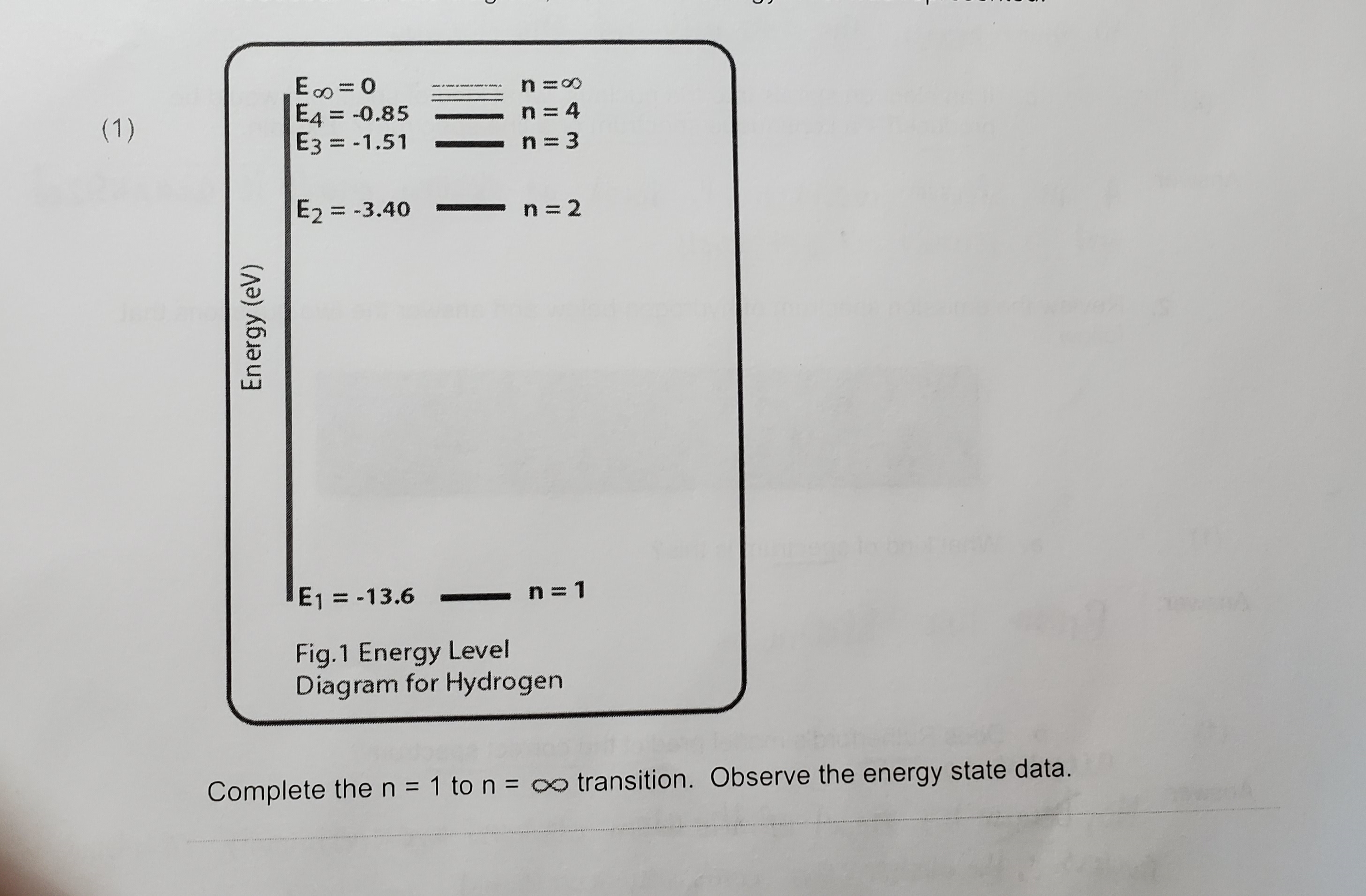
/2 1. What was the evidence that the cathode rays were particles with charge and mass? Answer:7. In a hydrogen atom, the electron jumps from the n = 1 level to the n = 4 level. (1 ) a . During the transition from n = 1 to n = 4, is a photon emitted or absorbed? Answer: (1 ) b . What is the change in energy of the electron? Answer: (1 ) c. Identify the transition on the energy level diagram below or state in words what the transition is.E. = 0 n =0 (1 ) E4 = -0.85 n = 4 E3= -1.51 n = 3 E2 = -3.40 n = 2 Energy (ev) E1 = -13.6 n =1 Fig.1 Energy Level Diagram for Hydrogen Complete the n = 1 to n = transition. Observe the energy state data
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


