Question: 2. (10 pt) Consider the bonding orbitals between H and N. If one sigma bonding orbital takes the following form: (H) +0.76 w2p (N). We
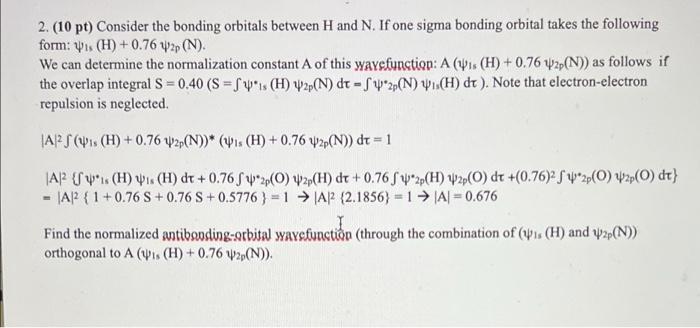
2. (10 pt) Consider the bonding orbitals between H and N. If one sigma bonding orbital takes the following form: (H) +0.76 w2p (N). We can determine the normalization constant A of this waysfunction: A (is (H) +0.76 2p(N)) as follows if the overlap integral S=0.40 (S=f1s (H) W2p(N) dt -f2p(N) Wis(H) dt). Note that electron-electron repulsion is neglected. A2 S (Ws (H) +0.76 2p(N))* (is (H) +0.76 42p(N)) dt = 1 AP 1 (H) . (H) dt+ 0.76 f2p(0) 2p(H) dt +0.76 f2p(H) 2p(O) dt +(0.76)22p(0)2p(0) dt} = |A2 {1+0.76 S+0.76 S+0.5776}=1 A2 (2.1856)=1 |A| = 0.676 Find the normalized antibanding-orbital waysfunction (through the combination of (1. (H) and 2p(N)) orthogonal to A (is (H) + 0.76 W2p(N))
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


