Question: 2. (25 pts) Let's think about the engine as shown below with 1mol of a monoatomic ideal gas (Cv=1.5R,Cp=2.5R ). 1>2 is isobar (i.e., constant
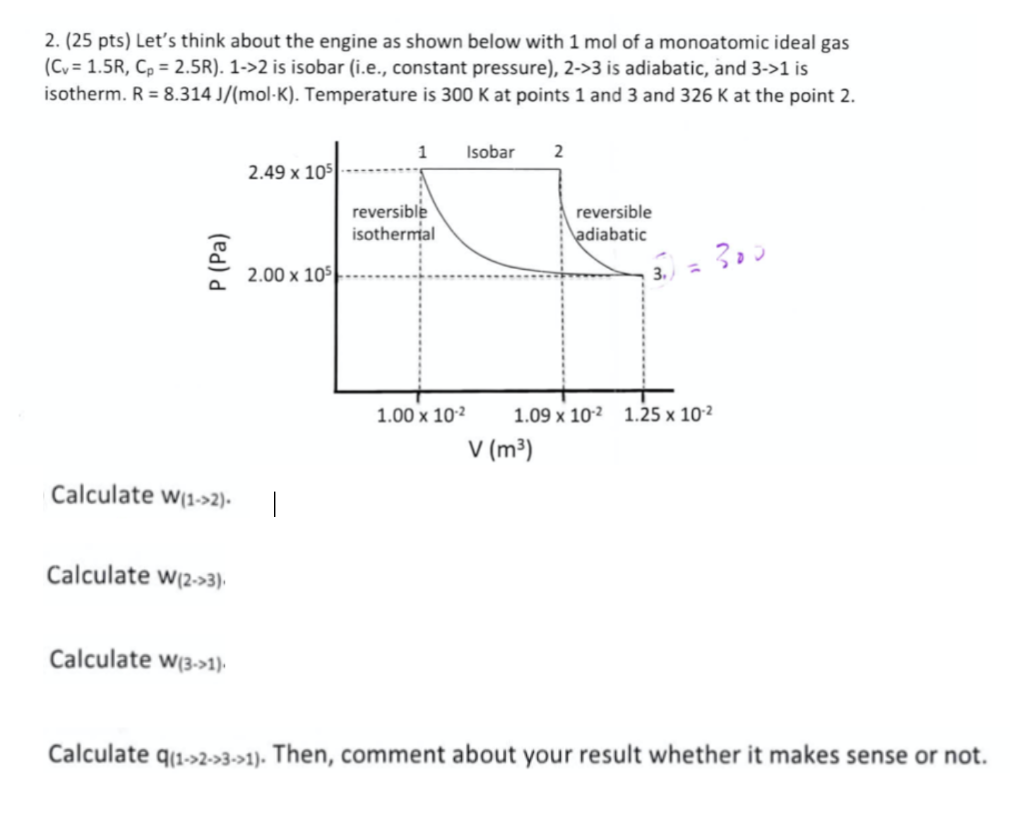
2. (25 pts) Let's think about the engine as shown below with 1mol of a monoatomic ideal gas (Cv=1.5R,Cp=2.5R ). 1>2 is isobar (i.e., constant pressure), 2>3 is adiabatic, and 3>1 is isotherm. R=8.314J/(molK). Temperature is 300K at points 1 and 3 and 326K at the point 2 . Calculate w(1>2) Calculate w(2>3) Calculate w(3>>) Calculate q(1>2>3>1). Then, comment about your result whether it makes sense or not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


