Question: 2. (40 points) Consider a reversible, gas-phase, elementary reaction A + B - C carried out in a plug flow reactor (CSTR): (a) (15 points)
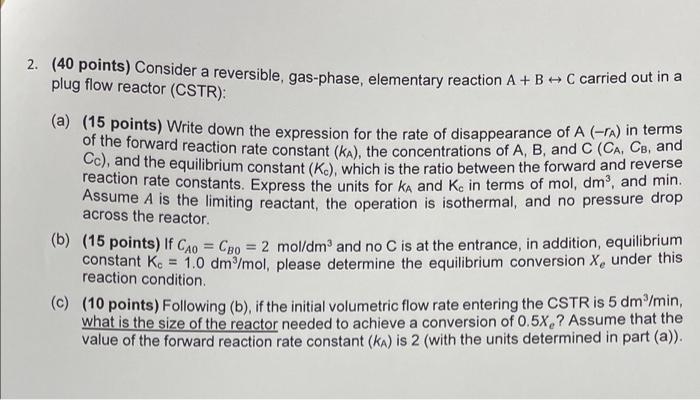
2. (40 points) Consider a reversible, gas-phase, elementary reaction A + B - C carried out in a plug flow reactor (CSTR): (a) (15 points) Write down the expression for the rate of disappearance of A (ra) in terms of the forward reaction rate constant (ka), the concentrations of A, B, and C (CA. Ce, and Cc), and the equilibrium constant (K.), which is the ratio between the forward and reverse reaction rate constants. Express the units for ka and Ke in terms of mol, dm, and min. Assume A is the limiting reactant, the operation is isothermal, and no pressure drop across the reactor. (b) (15 points) If Cho = Cpo = 2 mol/dm' and no C is at the entrance, in addition, equilibrium constant Kc = 1,0 dm-/mol , please determine the equilibrium conversion X, under this reaction condition. (C) (10 points) Following (b), if the initial volumetric flow rate entering the CSTR is 5 dm /min, what is the size of the reactor needed to achieve a conversion of 0.5x,? Assume that the value of the forward reaction rate constant (KA) is 2 (with the units determined in part (a))
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


