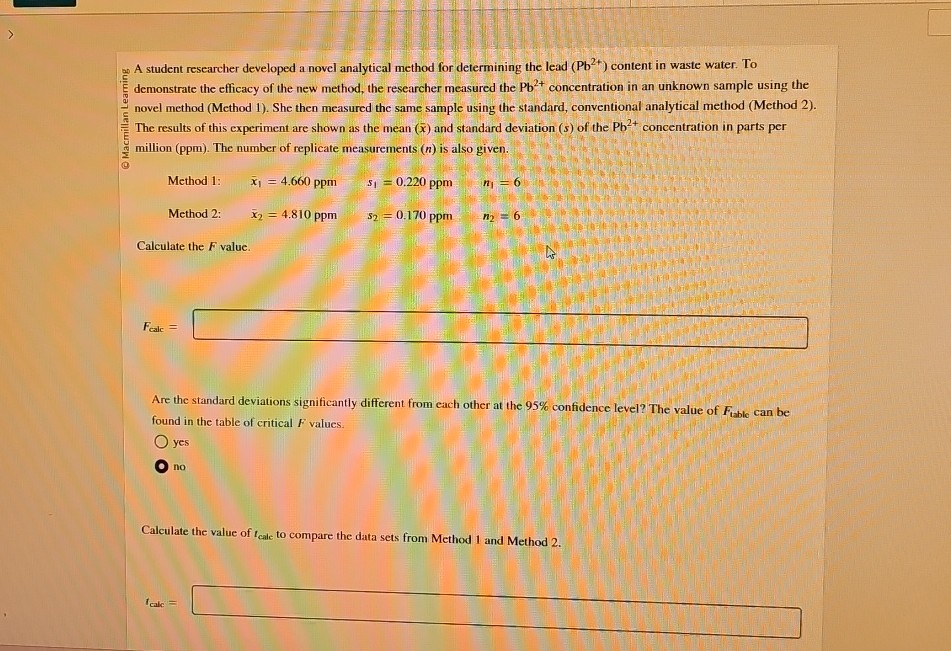
Question: 2 5 A student researcher developed a novel analytical method for determining the lead ( P b 2 + ) content in waste water. To
A student researcher developed a novel analytical method for determining the lead content in waste water. To demonstrate the efficacy of the new method, the researcher measured the concentration in an unknown sample using the novel method Method She then measured the same sample using the standard, conventional analytical method Method The results of this experiment are shown as the mean and standard deviation of the concentration in parts per million ppm The number of replicate measurements is also given.
Method :
Method :
Calculate the value.
Are the standard deviations significantly different from each other at the confidence level? The value of can be found in the table of critical values.
yes
no
Calculate the value of to compare the data sets from Method and Method
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


