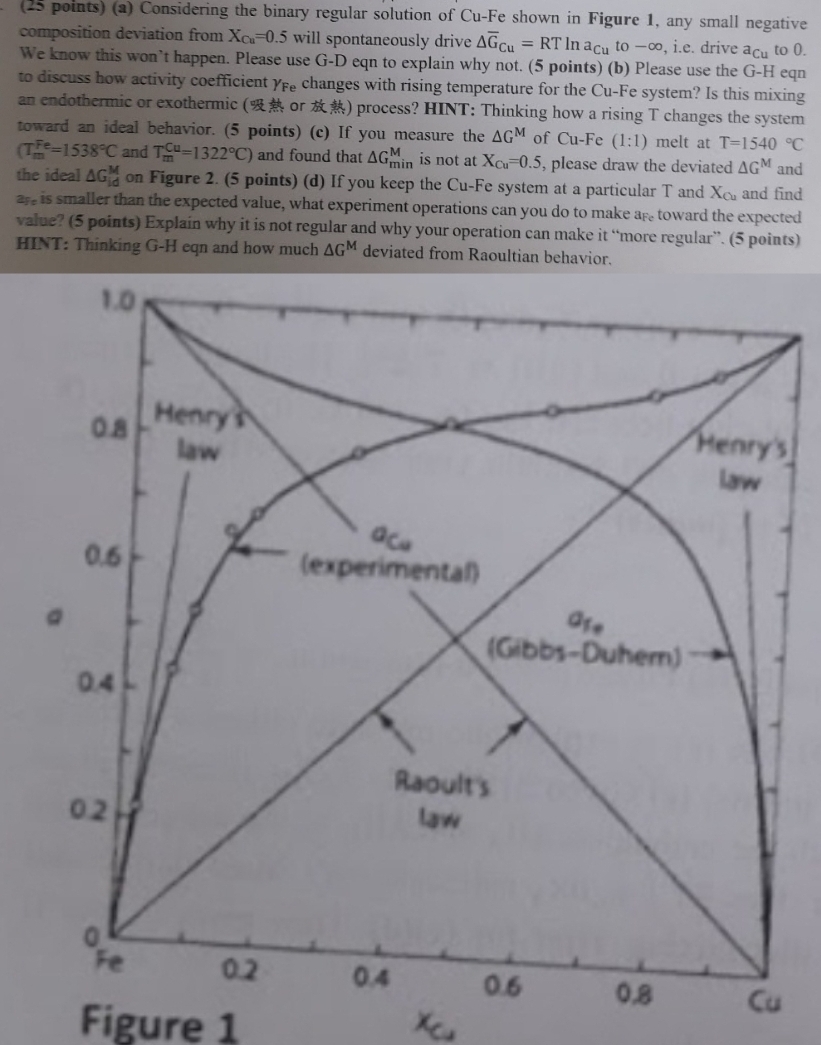
Question: ( 2 5 points ) ( a ) Considering the binary regular solution of C u - F e shown in Figure 1 , any
pointsa Considering the binary regular solution of shown in Figure any small negative composition deviation from will spontaneously drive to ie drive to We know this won't happen. Please use GD eqn to explain why not. pointsb Please use the GH eqn to discuss how activity coefficient changes with rising temperature for the system? Is this mixing an endothermic or exothermic or process? HINT: Thinking how a rising changes the system toward an ideal behavior. pointsc If you measure the of : melt at and : and found that is not at please draw the deviated and the ideal on Figure pointsd If you keep the CuFe system at a particular and and find is smaller than the expected value, what experiment operations can you do to make toward the expected value? points Explain why it is not regular and why your operation can make it "more regular". points HINT: Thinking GH eqn and how much deviated from Raoultian behavior.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


