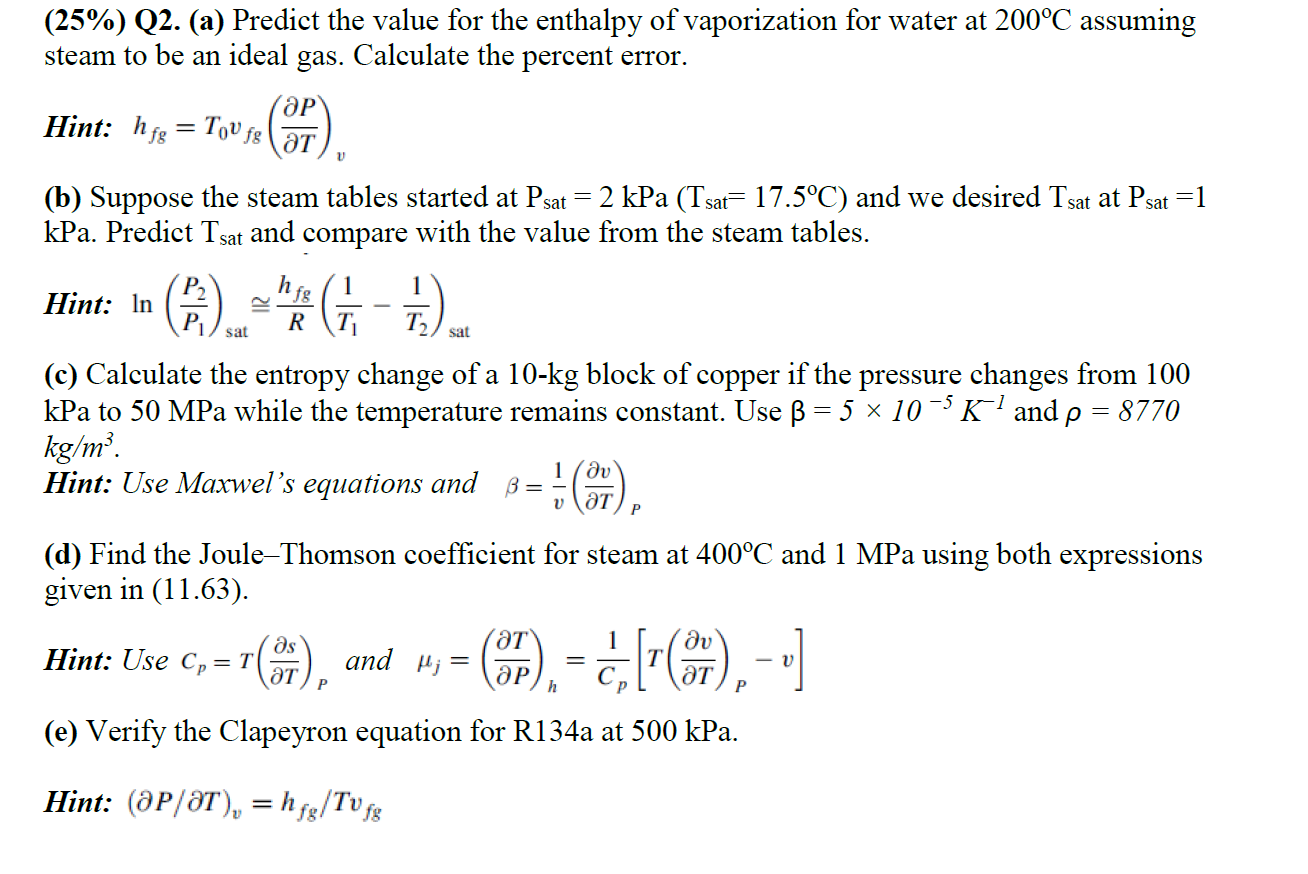
Question: ( 2 5 % ) Q 2 . ( a ) Predict the value for the enthalpy of vaporization for water at 2 0 0
Qa Predict the value for the enthalpy of vaporization for water at assuming Qa Predict the value for the enthalpy of vaporization for water at assuming
steam to be an ideal gas. Calculate the percent error.
Hint:
b Suppose the steam tables started at kPa and we desired at
kPa. Predict and compare with the value from the steam tables.
Hint: ~
c Calculate the entropy change of a kg block of copper if the pressure changes from
kPa to MPa while the temperature remains constant. Use and
Hint: Use Maxwel's equations and
d Find the JouleThomson coefficient for steam at and MPa using both expressions
given in
Hint: Use and
e Verify the Clapeyron equation for Ra at kPa.
Hint: elT
steam to be an ideal gas. Calculate the percent error.
Hint:
b Suppose the steam tables started at kPa and we desired at
kPa. Predict and compare with the value from the steam tables.
Hint: ~
c Calculate the entropy change of a kg block of copper if the pressure changes from
kPa to MPa while the temperature remains constant. Use and
Hint: Use Maxwel's equations and
d Find the JouleThomson coefficient for steam at and MPa using both expressions
given in
Hint: Use and
e Verify the Clapeyron equation for Ra at kPa.
Hint: elT
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


