Question: 2. A 275mg sample of a nonelectrolyte compound was dissolved in water to produce 10.0mL of a solution at 25C. The osmotic pressure of this
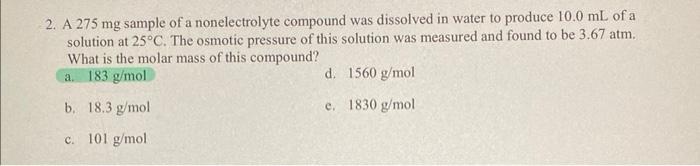
2. A 275mg sample of a nonelectrolyte compound was dissolved in water to produce 10.0mL of a solution at 25C. The osmotic pressure of this solution was measured and found to be 3.67atm. What is the molar mass of this compound? a. 183g/mol d. 1560g/mol b. 18.3g/mol e. 1830g/mol c. 101g/mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


