Question: 2. A fuel analyzing 27% CO, 12% CO2, 2% H2, 5% CH4 and 54% N and 98 kPa and saturated with water is burned
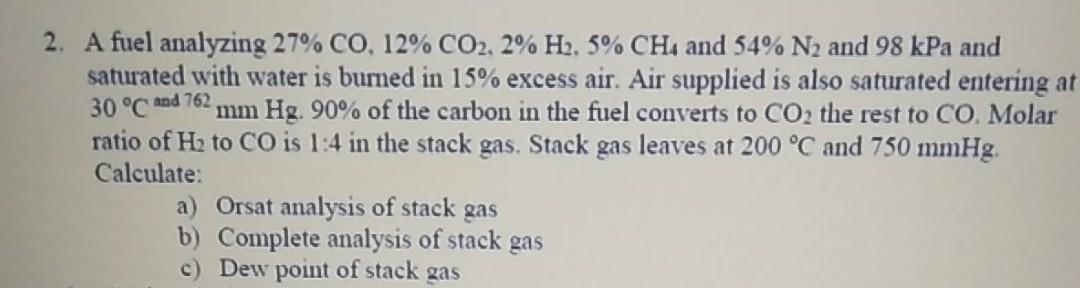
2. A fuel analyzing 27% CO, 12% CO2, 2% H2, 5% CH4 and 54% N and 98 kPa and saturated with water is burned in 15% excess air. Air supplied is also saturated entering at 30 C and 762, mm Hg. 90% of the carbon in the fuel converts to CO2 the rest to CO. Molar ratio of H to CO is 1:4 in the stack gas. Stack gas leaves at 200 C and 750 mmHg. Calculate: a) Orsat analysis of stack gas b) Complete analysis of stack gas c) Dew point of stack gas
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


