Question: 2. A gas sample is known to be a mixture of ethane and butane. A bulb of 200.0 cm3 capacity is filled with the gas
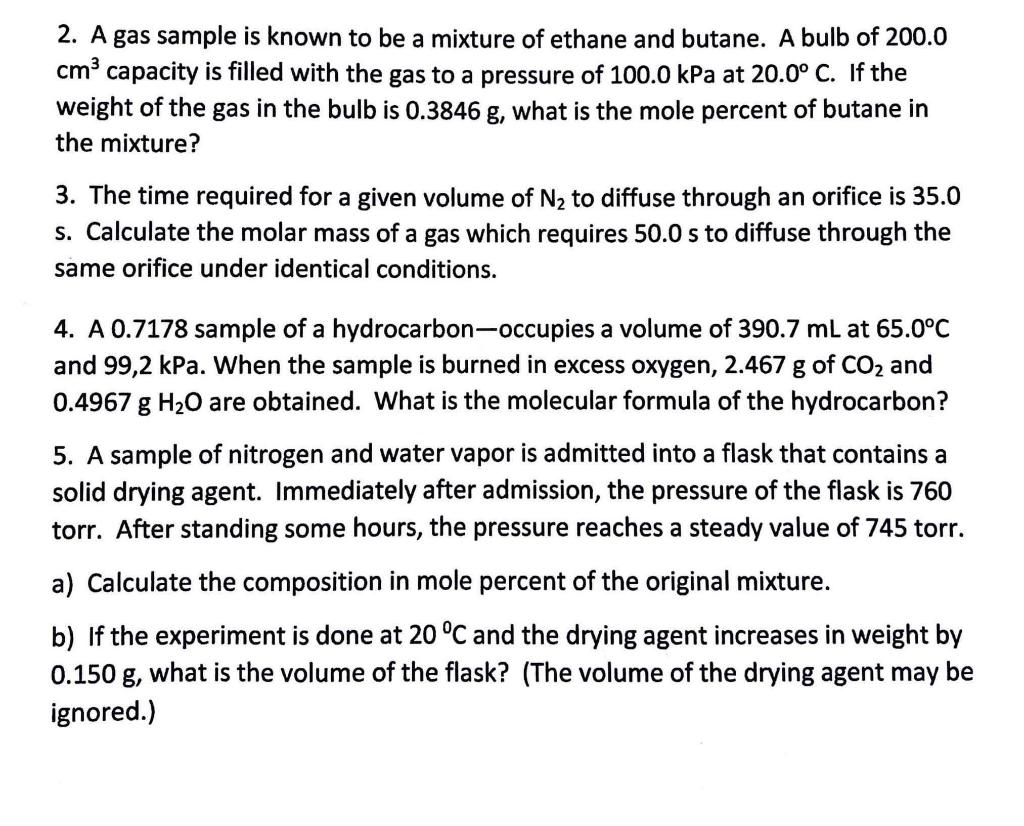
2. A gas sample is known to be a mixture of ethane and butane. A bulb of 200.0 cm3 capacity is filled with the gas to a pressure of 100.0kPa at 20.0C. If the weight of the gas in the bulb is 0.3846g, what is the mole percent of butane in the mixture? 3. The time required for a given volume of N2 to diffuse through an orifice is 35.0 s. Calculate the molar mass of a gas which requires 50.0s to diffuse through the same orifice under identical conditions. 4. A 0.7178 sample of a hydrocarbon-occupies a volume of 390.7mL at 65.0C and 99,2kPa. When the sample is burned in excess oxygen, 2.467g of CO2 and 0.4967gH2O are obtained. What is the molecular formula of the hydrocarbon? 5. A sample of nitrogen and water vapor is admitted into a flask that contains a solid drying agent. Immediately after admission, the pressure of the flask is 760 torr. After standing some hours, the pressure reaches a steady value of 745 torr. a) Calculate the composition in mole percent of the original mixture
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


