Question: 2- A liquid solution of ethane (1) and propane (2) is at equilibrium with its vapor at P = 20 bar and T = 300K.
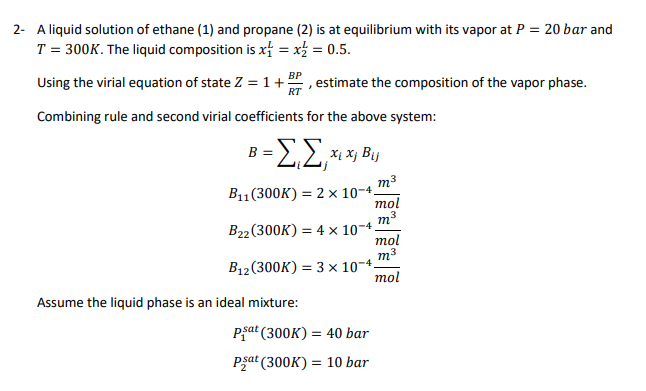
2- A liquid solution of ethane (1) and propane (2) is at equilibrium with its vapor at P = 20 bar and T = 300K. The liquid composition is x1 = x3 = 0.5. Using the virial equation of state 2 = 1 + BP estimate the composition of the vapor phase. RT Combining rule and second virial coefficients for the above system: B 3 Xix; Bij m3 = = B11 (300K) = 2 x 10-4 mol m3 B22(300K) = 4 x 10-4 mol m3 B12(300K) = 3 x 10-4 mol Assume the liquid phase is an ideal mixture: psat (300K) = 40 bar Pat (300K) = 10 bar
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


